Physicians’ Experience with COVID-19 Vaccination: A Survey Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Survey Instruments
2.4. Ethics
2.5. Statistical Analysis
3. Results
3.1. General Data
3.2. Side Effects Reported after COVID-19 Vaccination
3.3. The Impact of the COVID-19 Vaccination
3.4. The Experience of the COVID-19 Disease
3.5. Serological Response to the COVID-19 Vaccination
4. Discussion
Limitations
5. Conclusions
Strengths and Limitations of This Study
- The research presents data on vaccine efficiency and safety in one of the European countries with lowest rate of vaccination.
- It is important to note that none of the COVID-19 cases was declared after full vaccination and within 6 months after vaccination.
- Very high anti-spike SARS-CoV2 IgG antibodies titers were more frequently observed in vaccinated physicians who previously obtained natural immunity.
- The online questionnaire distribution is the one limitation of this research.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dima, A.; Balaban, D.; Jurcut, C.; Berza, I.; Jurcut, R.; Jinga, M. Perceptions of Romanian Physicians on Lockdowns for COVID-19 Prevention. Healthcare 2021, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Alina, D.; Vasile, B.; Ciprian, J.; Ioana, B.; Ruxandra, J.; Mariana, J. Physicians’ Perspectives on COVID-19: An International Survey. Healthcare 2020, 8, 250. [Google Scholar] [CrossRef]
- Kozak, A.; Nienhaus, A. COVID-19 vaccination: Status and willingness to be vaccinated among employees in health and welfare care in germany. Int. J. Environ. Res. Public. Health 2021, 18, 6688. [Google Scholar] [CrossRef]
- Marinos, G.; Lamprinos, D.; Georgakopoulos, P.; Patoulis, G.; Vogiatzi, G.; Damaskos, C.; Papaioannou, A.; Sofroni, A.; Pouletidis, T.; Papagiannis, D.; et al. Reported covid-19 vaccination coverage and associated factors among members of athens medical association: Results from a cross-sectional study. Vaccines 2021, 9, 1134. [Google Scholar] [CrossRef]
- Robbins, T.; Kyrou, I.; Clark, C.; Sharma, K.; Laird, S.; Berry, L.; Morgan, N.; Patel, K.; Sankar, S.; Randeva, H. Healthcare staff perceptions following inoculation with the bnt162b2 mrna covid-19 vaccine at university hospitals coventry & warwickshire nhs trust. Int. J. Environ. Res. Public Health 2021, 18, 9378. [Google Scholar] [CrossRef]
- Biswas, N.; Mustapha, T.; Khubchandani, J.; Price, J.H. The Nature and Extent of COVID-19 Vaccination Hesitancy in Healthcare Workers. J. Community Health 2021, 46, 1244–1251. [Google Scholar] [CrossRef]
- Gagneux-Brunon, A.; Detoc, M.; Bruel, S.; Tardy, B.; Rozaire, O.; Frappe, P.; Botelho-Nevers, E. Intention to get vaccinations against COVID-19 in French healthcare workers during the first pandemic wave: A cross-sectional survey. J. Hosp. Infect. 2021, 108, 168. [Google Scholar] [CrossRef]
- Mustapha, T.; Khubchandani, J.; Biswas, N. COVID-19 vaccination hesitancy in students and trainees of healthcare professions: A global assessment and call for action. Brain Behav. Immun. Health 2021, 16, 100289. [Google Scholar] [CrossRef]
- Bolsewicz, K.T.; Steffens, M.S.; Bullivant, B.; King, C.; Beard, F. “To Protect Myself, My Friends, Family, Workmates and Patients … and to Play My Part”: COVID-19 Vaccination Perceptions among Health and Aged Care Workers in New South Wales, Australia. Int. J. Environ. Res. Public Health 2021, 18, 8954. [Google Scholar] [CrossRef]
- Li, M.; Luo, Y.; Watson, R.; Zheng, Y.; Ren, J.; Tang, J.; Chen, Y. Healthcare workers’ (HCWs) attitudes and related factors towards COVID-19 vaccination: A rapid systematic review. Postgrad Med. J. 2021, 1, 1–7. [Google Scholar] [CrossRef]
- Eysenbach, G. Improving the Quality of Web Surveys: The Checklist for Reporting Results of Internet E-Surveys (CHERRIES). J. Med. Internet Res. 2004, 6, e34. [Google Scholar] [CrossRef]
- Streinu-Cercel, A.; Apostolescu, C.; Săndulescu, O.; Oțelea, D.; Streinu-Cercel, A.; Vlaicu, O.; Paraschiv, S.; Benea, O.E.; Bacruban, R.; Nițescu, M.; et al. Sars-COV-2 in Romania–analysis of the first confirmed case and evolution of the pandemic in Romania in the first three months. Germs 2020, 10, 132–134. [Google Scholar] [CrossRef] [PubMed]
- HOTARARE 293 10/03/2021-Portal Legislativ. Available online: http://legislatie.just.ro/Public/DetaliiDocumentAfis/239080 (accessed on 18 July 2021).
- Craxì, L.; Casuccio, A.; Amodio, E.; Restivo, V. Who Should Get COVID-19 Vaccine First? A Survey to Evaluate Hospital Workers’ Opinion. Vaccines 2021, 9, 189. [Google Scholar] [CrossRef]
- Coronavirus (COVID-19) Vaccinations-Statistics and Research-Our World in Data. Available online: https://ourworldindata.org/covid-vaccinations?country=OWID_WRL (accessed on 1 September 2021).
- Yılmaz, M.; Sahin, M.K. Parents’ willingness and attitudes concerning the COVID-19 vaccine: A cross-sectional study. Int. J. Clin. Pract. 2021, 75, e14364. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-W.; Wen, W.; Wang, N.; Zhou, M.-Y.; Wang, C.-Y.; Ni, J.; Jiang, J.-J.; Zhang, X.-W.; Feng, Z.-H.; Cheng, Y.-R. COVID-19 Vaccination Acceptance Among Healthcare Workers and Non-healthcare Workers in China: A Survey. Front. Public Health 2021, 9, 1–8. [Google Scholar] [CrossRef]
- Xin, M.; Luo, S.; She, R.; Chen, X.; Li, L.; Li, L.; Chen, X.; Lau, J.T.F. The Impact of Social Media Exposure and Interpersonal Discussion on Intention of COVID-19 Vaccination among Nurses. Vaccines 2021, 9, 1204. [Google Scholar] [CrossRef]
- Lindholt, M.F.; Jørgensen, F.; Bor, A.; Petersen, M.B. Public acceptance of COVID-19 vaccines: Cross-national evidence on levels and individual-level predictors using observational data. BMJ Open 2021, 11, e048172. [Google Scholar] [CrossRef]
- Riccò, M.; Ferraro, P.; Peruzzi, S.; Balzarini, F.; Ranzieri, S. Mandate or not mandate: Knowledge, attitudes, and practices of italian occupational physicians towards SARS-CoV-2 immunization at the beginning of vaccination campaign. Vaccines 2021, 9, 889. [Google Scholar] [CrossRef] [PubMed]
- Pataka, A.; Kotoulas, S.; Stefanidou, E.; Grigoriou, I.; Tzinas, A.; Tsiouprou, I.; Zarogoulidis, P.; Courcoutsakis, N.; Argyropoulou, P. Acceptability of Healthcare Professionals to Get Vaccinated against COVID-19 Two Weeks before Initiation of National Vaccination. Medicina 2021, 57, 611. [Google Scholar] [CrossRef]
- Al-Sanafi, M.; Sallam, M. Psychological Determinants of COVID-19 Vaccine Acceptance among Healthcare Workers in Kuwait: A Cross-Sectional Study Using the 5C and Vaccine Conspiracy Beliefs Scales. Vaccines 2021, 9, 701. [Google Scholar] [CrossRef] [PubMed]
- Hughes, K.; Gogineni, V.; Lewis, C.; Deshpande, A. Considerations for fair prioritization of COVID-19 vaccine and its mandate among healthcare personnel. Curr. Med. Res. Opin. 2021, 37, 907–909. [Google Scholar] [CrossRef]
- Halim, M. COVID-19 Vaccination Efficacy and Safety Literature Review. J. Immunol. Allergy 2021, 3, 1–19. [Google Scholar] [CrossRef]
- Brouqui, P.; Colson, P.; Melenotte, C.; Houhamdi, L.; Bedotto, M.; Devaux, C.; Gautret, P.; Million, M.; Parola, P.; Stoupan, D.; et al. COVID-19 re-infection. Eur. J. Clin. Investig. 2021, 51, 4–8. [Google Scholar] [CrossRef]
- Yassi, A.; Grant, J.M.; Lockhart, K.; Barker, S.; Sprague, S.; Okpani, A.I.; Wong, T.; Daly, P.; Henderson, W.; Lubin, S.; et al. Infection control, occupational and public health measures including mRNA-based vaccination against SARS-CoV-2 infections to protect healthcare workers from variants of concern: A 14-month observational study using surveillance data. PLoS ONE 2021, 16, e0254920. [Google Scholar] [CrossRef] [PubMed]
- Štěpánek, L.; Janošíková, M.; Nakládalová, M.; Štěpánek, L.; Boriková, A.; Vildová, H. Motivation to COVID-19 Vaccination and Reasons for Hesitancy in Employees of a Czech Tertiary Care Hospital: A Cross-Sectional Survey. Vaccines 2021, 9, 863. [Google Scholar] [CrossRef] [PubMed]
- Amodio, E.; Capra, G.; Casuccio, A.; Grazia, S.; Genovese, D.; Pizzo, S.; Calamusa, G.; Ferraro, D.; Giammanco, G.; Vitale, F.; et al. Antibodies Responses to SARS-CoV-2 in a Large Cohort of Vaccinated Subjects and Seropositive Patients. Vaccines 2021, 9, 714. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, F.; Buonfrate, D.; Moro, L.; Rodari, P.; Piubelli, C.; Caldrer, S.; Riccetti, S.; Sinigaglia, A.; Barzon, L. Antibody Response to the BNT162b2 mRNA COVID-19 Vaccine in Subjects with Prior SARS-CoV-2 Infection. Viruses 2021, 13, 422. [Google Scholar] [CrossRef]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Rzymski, P.; Camargo, C.A.; Fal, A.; Flisiak, R.; Gwenzi, W.; Kelishadi, R.; Leemans, A.; Nieto, J.J.; Ozen, A.; Perc, M.; et al. COVID-19 Vaccine Boosters: The Good, the Bad, and the Ugly. Vaccines 2021, 9, 1299. [Google Scholar] [CrossRef]
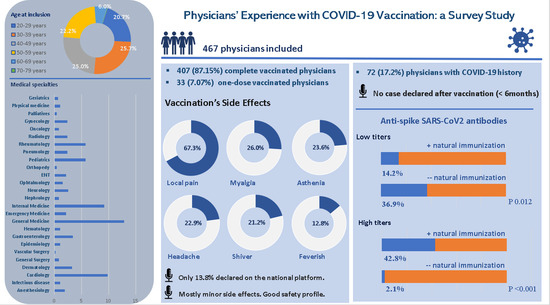
| Respondents, n (%) | |
|---|---|
| COVID19 vaccination (Q8) | |
| Yes, both doses | 407/467 (87.15%) |
| Yes, one dose | 33/467 (7.07%) |
| No | 27/467 (5.78%) |
| Reasons for not being vaccinated (Q9) | |
| History of allergies | 3/24 (12.50%) |
| History of anaphylactic reaction | 1/24 (4.17%) |
| Recent SARS-CoV2 infection | 8/24 (33.33%) |
| Do not trust the vaccine efficiency | 5/24 (20.83%) |
| Something else | 9/24 (37.5%) |
| Type of COVID19 vaccine received (Q10) | |
| Pfizer—BioNTech (Comirnaty)—BNT162b2 | 413/420 (98.33%) |
| Moderna—mRNA-1273 | 3/420 (0.71%) |
| AstraZeneca/Oxford—AZD1222 | 4/420 (0.95%) |
| Period for the COVID19 vaccination (Q11) | |
| December 2020 | 25/420 (5.95%) |
| January 2021 | 332/420 (79.05%) |
| February 2021 | 38/420 (9.05%) |
| Marth 2021 | 24/420 (5.71%) |
| April 2021 | 1/420 (0.24%) |
| Respondents, n (%) | |
|---|---|
| Side effects after the second vaccine dose (Q16) | |
| I did not receive yet the second vaccine dose | 29/419 (6.92%) |
| None | 47/419 (11.22%) |
| Local cutaneous changes at injection side | 15/419 (3.58%) |
| Pain at injection site | 282/419 (67.30%) |
| Easy-moderate allergic reaction | 3/419 (0.72%) |
| Severe allergic reaction | 0/419 (0.00%) |
| Cutaneous eruptions | 2/419 (0.48%) |
| Important asthenia | 99/419 (23.63%) |
| Sleepiness | 97/419 (23.15%) |
| Insomnia | 26/419 (6.21%) |
| Feverish | 54/419 (12.89%) |
| Fever | 37/419 (8.83%) |
| Shiver | 89/419 (21.24%) |
| Myalgia | 109/419 (26.01%) |
| Appetit loss | 10/419 (2.39%) |
| Nausea/vomiting | 24/419 (5.73%) |
| Diarrhea | 3/419 (0.72%) |
| Headache | 96/419 (22.91%) |
| Drowsiness | 28/419 (6.68%) |
| Tinnitus | 1/419 (0.24%) |
| Vertigo | 13/419 (3.10%) |
| Odynophagia | 4/419 (0.95%) |
| Cough | 1/419 (0.24%) |
| Palpitations | 15/419 (3.58%) |
| Increased value of arterial tension | 6/419 (1.43%) |
| Adenopathy axillar/supraclavicular | 36/419 (8.59%) |
| Something else (were completed: hypothermia, trigemini neuralgia, Raynaud phenomenon, arthralgia, trouble of concentration, zona zoster) | 26/419 (6.21%) |
| The period after the second vaccine dose in which the side effects appeared (Q17) | |
| I did not receive yet the second vaccine dose | 28/415 (6.75%) |
| Does not apply, I did not have side effects | 46/415 (11.08%) |
| Less than 24 hours | 159/415 (38.31%) |
| 1–3 days | 149/415 (35.90%) |
| 3–7 days | 19/415 (4.58%) |
| 1–2 weeks | 4/415 (0.96%) |
| 2–4 weeks | 4/415 (0.96%) |
| 1–2 months | 4/415 (0.96%) |
| More than 2 months | 0/415 (0.00%) |
| Something else | 2/415 (0.48%) |
| Treatment for the COVID-19 vaccine’s side effects (Q19) | |
| I did not receive yet the second vaccine dose | 28/417 (6.71%) |
| Does not apply, I did not have side effects | 50/417 (11.99%) |
| Nothing, spontaneous improvement | 152/417 (36.45%) |
| Antihistamines | 8/417 (1.92%) |
| Paracetamol | 147/417 (35.25%) |
| Nonsteroidal anti-inflammatory drugs | 75/417 (17.99%) |
| Corticosteroids | 1/417 (0.24%) |
| Yes, something else | 15/417 (3.60%) |
| Respondents, n (%) | |
|---|---|
| The COVID-19 side effect reported on the national dedicated platform (Q20) | |
| Does not apply, I did not have side effects | 61/421 (14.49%) |
| Yes, I did report the side effects | 50/421 (11.88%) |
| No, I did not report the side effects | 310/421 (73.63%) |
| The necessity of medical sick leave for the side effects secondary to COVID-19 vaccination (Q21) | |
| No, there are only mild side effects | 176/421 (41.81%) |
| Yes, very rare sick leave might be needed | 203/421 (48.22%) |
| Yes, quite frequent sick leave is needed | 37/421 (8.79%) |
| Yes, sick leave after vaccination is usually needed | 5/421 (1.19%) |
| Anti-Spike SARS-CoV2 antibodies’ titer after complete vaccination (Q22) | |
| Does not apply, I did not make the determination | 259/419 (61.81%) |
| Negative titer, under the laboratory titer | 2/419 (0.48%) |
| Positive titer, 1–100 times the laboratory limit | 44/419 (10.50%) |
| Positive titer, 100–1000 times the laboratory limit | 54/419 (12.89%) |
| Positive titer, 1000–5000 times the laboratory limit | 40/419 (9.55%) |
| Positive titer, 5000–10,000 times the laboratory limit | 8/419 (1.91%) |
| Positive titer, more than 10,000 times the laboratory limit | 12/419 (2.86%) |
| Moment after vaccination for the anti-Spike SARS-CoV2 antibodies’ determination (Q23) | |
| Does not apply, I did not make the determination | 259/418 (61.96%) |
| Less than 2 weeks after the second vaccination dose | 46/418 (11.00%) |
| 2–4 weeks after the second vaccination dose | 89/418 (21.29%) |
| 1–2 months after the second vaccination dose | 23/418 (5.50%) |
| 2–3 months after the second vaccination dose | 1/418 (0.24%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dima, A.; Jurcut, C.; Balaban, D.V.; Gheorghita, V.; Jurcut, R.; Dima, A.C.; Jinga, M. Physicians’ Experience with COVID-19 Vaccination: A Survey Study. Healthcare 2021, 9, 1746. https://0-doi-org.brum.beds.ac.uk/10.3390/healthcare9121746
Dima A, Jurcut C, Balaban DV, Gheorghita V, Jurcut R, Dima AC, Jinga M. Physicians’ Experience with COVID-19 Vaccination: A Survey Study. Healthcare. 2021; 9(12):1746. https://0-doi-org.brum.beds.ac.uk/10.3390/healthcare9121746
Chicago/Turabian StyleDima, Alina, Ciprian Jurcut, Daniel Vasile Balaban, Valeriu Gheorghita, Ruxandra Jurcut, Augustin Catalin Dima, and Mariana Jinga. 2021. "Physicians’ Experience with COVID-19 Vaccination: A Survey Study" Healthcare 9, no. 12: 1746. https://0-doi-org.brum.beds.ac.uk/10.3390/healthcare9121746