The Modern and Digital Transformation of Oral Health Care: A Mini Review
Abstract
:1. Introduction
2. Augmented and Virtual Reality
3. Tele-Dentistry with Remote Consultation
4. Additive Manufacturing
4.1. Additive Manufacturing for Surgical Guide in Implant Rehabilitation
4.1.1. Static Guided Systems
4.1.2. Dynamic Navigation System (DNS)
5. Artificial Intelligence (AI)
6. Ethical Issues and Challenges in AR/VR and AI
7. Digital Oral Health Records
8. Digital Oral Scanner
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thomson, W.M.; Ma, S. An ageing population poses dental challenges. Singap. Dent. J. 2014, 35, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Favaretto, M.; Shaw, D.; De Clercq, E.; Joda, T.; Elger, B.S. Big Data and Digitalization in Dentistry: A Systematic Review of the Ethical Issues. Int. J. Environ. Res. Public Health 2020, 17, 2495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsen, W.; Kumar, S.; Shar, A.; Varoquiers, C.; Wiley, T.; Riley, W.T.; Atienza, A.A. Advancing the science of mHealth. J. Health Commun. 2012, 17 (Suppl. 1), 5–10. [Google Scholar] [CrossRef] [PubMed]
- Joda, T.; Bornstein, M.M.; Jung, R.E.; Ferrari, M.; Waltimo, T.; Zitzmann, N.U. Recent trends and future direction of dental research in the digital era. Int. J. Environ. Res. Public Health 2020, 17, 1987. [Google Scholar] [CrossRef] [Green Version]
- Rekow, E.D. Digital dentistry: The new state of the art—Is it disruptive or destructive? Dent. Mater. 2020, 36, 9–24. [Google Scholar] [CrossRef]
- Huang, T.K.; Yang, C.H.; Hsieh, Y.H.; Wang, J.C.; Hung, C.C. Augmented reality (AR) and virtual reality (VR) applied in dentistry. Kaohsiung J. Med. Sci. 2018, 34, 243–248. [Google Scholar] [CrossRef]
- Farronato, M.; Maspero, C.; Lanteri, V.; Fama, A.; Ferrati, F.; Pettenuzzo, A.; Farronato, D. Current state of the art in the use of augmented reality in dentistry: A systematic review of the literature. BMC Oral Health 2019, 19, 135. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, T.; Ikawa, T.; Shigeta, Y.; Kasama, S.; Ando, E.; Fukushima, S.; Suzuki, N. Virtual reality image applications for treatment planning in prosthodontic dentistry. In Proceedings of the MMVR 2011, Newport Beach, CA, USA, 8–12 February 2011; pp. 422–424. [Google Scholar]
- Raja’a, M.; Farid, F. Computer-based technologies in dentistry: Types and applications. J. Dent. 2016, 13, 215. [Google Scholar]
- Ayoub, A.; Pulijala, Y. The application of virtual reality and augmented reality in Oral & Maxillofacial Surgery. BMC Oral Health 2019, 19, 238. [Google Scholar]
- Ferro, A.S.; Nicholson, K.; Koka, S. Innovative Trends in Implant Dentistry Training and Education: A Narrative Review. J. Clin. Med. 2019, 8, 1618. [Google Scholar] [CrossRef] [Green Version]
- Pellegrino, G.; Mangano, C.; Mangano, R.; Ferri, A.; Taraschi, V.; Marchetti, C. Augmented reality for dental implantology: A pilot clinical report of two cases. BMC Oral Health 2019, 19, 158. [Google Scholar] [CrossRef] [PubMed]
- Chander, N.G. Augmented reality in prosthodontics. J. Indian Prosthodont. Soc. 2019, 19, 281. [Google Scholar] [CrossRef] [PubMed]
- Maspero, C.; Farronato, M.; Bellincioni, F.; Annibale, A.; Machetti, J.; Abate, A.; Cavagnetto, D. Three-dimensional evaluation of maxillary sinus changes in growing subjects: A retrospective cross-sectional study. Materials 2020, 13, 1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanteri, V.; Farronato, M.; Ugolini, A.; Cossellu, G.; Gaffuri, F.; Parisi, F.M.; Cavagnetto, D.; Abate, A.; Maspero, C. Volumetric Changes in the Upper Airways after Rapid and Slow Maxillary Expansion in Growing Patients: A Case-Control Study. Materials 2020, 13, 2239. [Google Scholar] [CrossRef] [PubMed]
- Maspero, C.; Farronato, M.; Bellincioni, F.; Cavagnetto, D.; Abate, A. Assessing mandibular body changes in growing subjects: A comparison of CBCT and reconstructed lateral cephalogram measurements. Sci. Rep. 2020, 10, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Farronato, M.; Cavagnetto, D.; Abate, A.; Cressoni, P.; Fama, A.; Maspero, C. Assessment of condylar volume and ramus height in JIA patients with unilateral and bilateral TMJ involvement: Retrospective case-control study. Clin. Oral Investig. 2020, 24, 2635–2643. [Google Scholar] [CrossRef]
- Maspero, C.; Abate, A.; Bellincioni, F.; Cavagnetto, D.; Lanteri, V.; Costa, A.; Farronato, M. Comparison of a tridimensional cephalometric analysis performed on 3T-MRI compared with CBCT: A pilot study in adults. Prog. Orthod. 2019, 20, 40. [Google Scholar] [CrossRef]
- Roy, E.; Bakr, M.M.; George, R. The need for virtual reality simulators in dental education: A review. Saudi Dent. J. 2017, 29, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Othman, N.I.; Ismail, H.U.; Mohammad, N.; Ghazali, N.; Alauddin, M.S. An Evaluation on Deep Caries Removal Method and Management Performed by Undergraduate Dental Students: A Malaysia Experience. Eur. J. Dent. 2020. [Google Scholar] [CrossRef]
- Towers, A.; Field, J.; Stokes, C.; Maddock, S.; Martin, N. A scoping review of the use and application of virtual reality in pre-clinical dental education. Br. Dent. J. 2019, 226, 358–366. [Google Scholar] [CrossRef]
- Besimo, C.E.; Zitzmann, N.U.; Joda, T. Digital Oral Medicine for the Elderly. Int. J. Environ. Res. Public Health 2020, 17, 2171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.A.; Omar, H. Teledentistry in practice: Literature review. Telemed. E-Health 2013, 19, 565–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jampani, N.D.; Nutalapati, R.; Dontula, B.S.K.; Boyapati, R. Applications of teledentistry: A literature review and update. J. Int. Soc. Prev. Community Dent. 2011, 1, 37. [Google Scholar] [PubMed]
- Martin, N.; Shahrbaf, S.; Towers, A.; Stokes, C.; Storey, C. Remote clinical consultations in restorative dentistry: A clinical service evaluation study. Br. Dent. J. 2020, 228, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Kumar, V.; Sharma, S.; Chawla, A.; Logani, A. Palliative dental care: Ignored dimension of dentistry amidst COVID-19 pandemic. Spec. Care Dent. 2020, 40, 613–615. [Google Scholar] [CrossRef]
- Crawford, E.; Taylor, N. The effective use of an e-dentistry service during the COVID-19 crisis. J. Orthod. 2020, 47, 330–337. [Google Scholar] [CrossRef]
- Santana, L.A.D.M.; Santos, M.A.L.D.; Albuquerque, H.I.M.D.; Costa, S.F.D.S.; Rezende-Silva, E.; Gercina, A.C.; Takeshita, W.M. Teledentistry in Brazil: A Viable Alternative during COVID-19 Pandemic. Rev. Bras. Epidemiol. 2020, 23, e200082. [Google Scholar] [CrossRef]
- Talla, P.K.; Levin, L.; Glogauer, M.; Cable, C.; Allison, P.J. Delivering dental care as we emerge from the initial phase of the COVID-19 pandemic: Teledentistry and face-to-face consultations in a new clinical world. Quintessence Int. 2020, 51, 672–677. [Google Scholar]
- Maspero, C.; Abate, A.; Cavagnetto, D.; El Morsi, M.; Fama, A.; Farronato, M. Available technologies, applications and benefits of teleorthodontics. A literature review and possible applications during the COVID-19 Pandemic. J. Clin. Med. 2020, 9, 1891. [Google Scholar] [CrossRef]
- Tofail, S.A.; Koumoulos, E.P.; Bandyopadhyay, A.; Bose, S.; O’Donoghue, L.; Charitidis, C. Additive manufacturing: Scientific and technological challenges, market uptake and opportunities. Mater. Today 2018, 21, 22–37. [Google Scholar] [CrossRef]
- Dawood, A.; Marti, B.M.; Sauret-Jackson, V.; Darwood, A. 3D printing in dentistry. Br. Dent. J. 2015, 219, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Hickel, R.; Reymus, M. 3D printing in dentistry—state of the art. Oper. Dent. 2020, 45, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.; Goodacre, B.J.; AlHelal, A.; Kattadiyil, M.T.; Richardson, P.M. Three-dimensional printing in contemporary fixed prosthodontics: A technique article. J. Prosthet. Dent. 2018, 119, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Park, T.; Chun, I.; Yun, K. The accuracy of a 3D printing surgical guide determined by CBCT and model analysis. J. Adv. Prosthodont. 2018, 10, 279–285. [Google Scholar] [CrossRef]
- Yeung, M.; Abdulmajeed, A.; Carrico, C.K.; Deeb, G.R.; Bencharit, S. Accuracy and precision of 3D-printed implant surgical guides with different implant systems: An in vitro study. J. Prosthet. Dent. 2020, 123, 821–828. [Google Scholar] [CrossRef]
- Unsal, G.S.; Turkyilmaz, I.; Lakhia, S. Advantages and limitations of implant surgery with CAD/CAM surgical guides: A literature review. J. Clin. Exp. Dent. 2020, 12, e409. [Google Scholar] [CrossRef]
- Greenberg, A.M. Digital technologies for dental implant treatment planning and guided surgery. Oral. Maxillofac. Surg. Clin. 2015, 27, 319–340. [Google Scholar] [CrossRef]
- Joda, T.; Ferrari, M.; Gallucci, G.O.; Wittneben, J.G.; Brägger, U. Digital technology in fixed implant prosthodontics. Periodontology 2000 2017, 73, 178–192. [Google Scholar] [CrossRef]
- Joda, T.; Zarone, F.; Ferrari, M. The complete digital workflow in fixed prosthodontics: A systematic review. BMC Oral Health 2017, 17, 124. [Google Scholar] [CrossRef]
- Colombo, M.; Mangano, C.; Mijiritsky, E.; Krebs, M.; Hauschild, U.; Fortin, T. Clinical applications and effectiveness of guided implant surgery: A critical review based on randomized controlled trials. BMC Oral Health 2017, 17, 150. [Google Scholar] [CrossRef]
- Joskowicz, L. Computer-aided surgery meets predictive, preventive, and personalized medicine. EPMA J. 2017, 8, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatakis, D.N.; Chien, H.H.; Parashis, A.O. Guided implant surgery risks and their prevention. Periodontol. 2000 2019, 81, 194–208. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, K.M.; Aras, M.A. Types of implant surgical guides in dentistry: A review. J. Oral Implantol. 2012, 38, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Gargallo-Albiol, J.; Barootchi, S.; Salomó-Coll, O.; Wang, H.L. Advantages and disadvantages of implant navigation surgery. A systematic review. Ann. Anat. Anat. Anz. 2019, 225, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mouhyi, J.; Salama, M.A.; Mangano, F.G.; Mangano, C.; Margiani, B.; Admakin, O. A novel guided surgery system with a sleeveless open frame structure: A retrospective clinical study on 38 partially edentulous patients with 1 year of follow-up. BMC Oral Health 2019, 19, 253. [Google Scholar] [CrossRef] [Green Version]
- Tallarico, M.; Meloni, S.M.; Martinolli, M.; Xhanari, E. Accuracy of sleeveless surgical templates-one-year randomized controlled trial. Clin. Oral Implant. Res. 2019, 30, 15. [Google Scholar] [CrossRef]
- Emery, R.W.; Merritt, S.A.; Lank, K.; Gibbs, J.D. Accuracy of dynamic navigation for dental implant placement–model-based evaluation. J. Oral Implantol. 2016, 42, 399–405. [Google Scholar] [CrossRef]
- Mandelaris, G.A.; Stefanelli, L.V.; DeGroot, B.S. Dynamic navigation for surgical implant placement: Overview of technology, key concepts, and a case report. Compend. Contin. Educ. Dent. 2018, 39, 614–621. [Google Scholar]
- Block, M.S.; Emery, R.W. Static or dynamic navigation for implant placement—choosing the method of guidance. J. Oral Maxillofac. Surg. 2016, 74, 269–277. [Google Scholar] [CrossRef]
- Block, M.S.; Emery, R.W.; Cullum, D.R.; Sheikh, A. Implant placement is more accurate using dynamic navigation. J. Oral Maxillofac. Surg. 2017, 75, 1377–1386. [Google Scholar] [CrossRef]
- Jorba-García, A.; Figueiredo, R.; González-Barnadas, A.; Camps-Font, O.; Valmaseda-Castellón, E. Accuracy and the role of experience in dynamic computer guided dental implant surgery: An in-vitro study. Med. Oralpatologia Oral Y Cir. Bucal 2019, 24, e76. [Google Scholar] [CrossRef] [PubMed]
- Golob Deeb, J.; Bencharit, S.; Carrico, C.K.; Lukic, M.; Hawkins, D.; Rener-Sitar, K.; Deeb, G.R. Exploring training dental implant placement using computer-guided implant navigation system for predoctoral students: A pilot study. Eur. J. Dent. Educ. 2019, 23, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Currie, G. Intelligent imaging: Anatomy of machine learning and deep learning. J. Nucl. Med. Technol. 2019, 47, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Park, W.J.; Park, J.B. History and application of artificial neural networks in dentistry. Eur. J. Dent. 2018, 12, 594. [Google Scholar] [CrossRef] [PubMed]
- Joda, T.; Waltimo, T.; Pauli-Magnus, C.; Probst-Hensch, N.; Zitzmann, N.U. Population-based linkage of big data in dental research. Int. J. Environ. Res. Public Health 2018, 15, 2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hung, K.; Yeung AW, K.; Tanaka, R.; Bornstein, M.M. Current Applications, Opportunities, and Limitations of AI for 3D Imaging in Dental Research and Practice. Int. J. Environ. Res. Public Health 2020, 17, 4424. [Google Scholar] [CrossRef]
- Schwendicke, F.; Elhennawy, K.; Paris, S.; Friebertshäuser, P.; Krois, J. Deep learning for caries lesion detection in near-infrared light transillumination images: A pilot study. J. Dent. 2020, 92, 103260. [Google Scholar] [CrossRef]
- Prados-Privado, M.; García Villalón, J.; Martínez-Martínez, C.H.; Ivorra, C.; Prados-Frutos, J.C. Dental Caries Diagnosis and Detection Using Neural Networks: A Systematic Review. J. Clin. Med. 2020, 9, 3579. [Google Scholar] [CrossRef]
- Hung, M.; Voss, M.W.; Rosales, M.N.; Li, W.; Su, W.; Xu, J.; Bounsanga, J.; Ruiz-Negrón, B.; Lauren, E.; Licari, F.W. Application of machine learning for diagnostic prediction of root caries. Gerodontology 2019, 36, 395–404. [Google Scholar] [CrossRef]
- Mallishery, S.; Chhatpar, P.; Banga, K.S.; Shah, T.; Gupta, P. The precision of case difficulty and referral decisions: An innovative automated approach. Clin. Oral Investig. 2019, 13, 1–7. [Google Scholar] [CrossRef]
- Orhan, K.; Bayrakdar, I.S.; Ezhov, M.; Kravtsov, A.; Özyürek, T.A. Evaluation of artificial intelligence for detecting periapical pathosis on cone-beam computed tomography scans. Int. Endod. J. 2020, 53, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Nozaki, K.; Gonda, T.; Ikebe, K. A system for designing removable partial dentures using artificial intelligence. Part 1. Classification of partially edentulous arches using a convolutional neural network. J. Prosthodont. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kunz, F.; Stellzig-Eisenhauer, A.; Zeman, F.; Boldt, J. Artificial intelligence in orthodontics. J. Orofac. Orthop. Fortschr. Der Kieferorthopädie 2020, 81, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Tay, F.R.; Gu, L. Application of Artificial Intelligence in Dentistry. J. Dent. Res. 2020, 29. [Google Scholar] [CrossRef]
- Shoukri, B.; Prieto, J.C.; Ruellas, A.; Yatabe, M.; Sugai, J.; Styner, M.; Zhu, H.; Huang, C.; Paniagua, B.; Aronovich, S.; et al. Minimally invasive approach for diagnosing TMJ osteoarthritis. J. Dent. Res. 2019, 98, 1103–1111. [Google Scholar] [CrossRef]
- Currie, G.; Hawk, K.E.; Rohren, E.M. Ethical principles for the application of artificial intelligence (AI) in nuclear medicine. Eur. J. Nucl. Med. Mol. Imaging. 2020, 47, 748–752. [Google Scholar] [CrossRef] [Green Version]
- Fiske, A.; Henningsen, P.; Buyx, A. Your robot therapist will see you now: Ethical implications of embodied artificial intelligence in psychiatry, psychology, and psychotherapy. J. Med. Internet Res. 2019, 21, e13216. [Google Scholar] [CrossRef]
- Sunny, S.; Baby, A.; James, B.L.; Balaji, D.; Rana, M.H.; Gurpur, P.; Skandarajah, A.; D’Ambrosio, M.; Ramanjinappa, R.D.; Mohan, S.P. A smart tele-cytology point-of-care platform for oral cancer screening. PLoS ONE 2019, 14, e0224885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopper, H.; Ranjan, M. What If Quantum Computer Combined with Artificial Intelligence? Sci. Insigt. 2019, 29, 48–51. [Google Scholar] [CrossRef]
- Sarma, S.D.; Deng, D.L.; Duan, L.M. Machine learning meets quantum physics. arXiv 2019, arXiv:1903.03516. [Google Scholar]
- Nanayakkara, S.; Zhou, X.; Spallek, H. Impact of big data on oral health outcomes. Oral Dis. 2019, 25, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Di Sanzo, M.; Cipolloni, L.; Borro, M.; La Russa, R.; Santurro, A.; Scopetti, M.; Simmaco, M.; Frati, P. Clinical applications of personalized medicine: A new paradigm and challenge. Curr. Pharm. Biotechnol. 2017, 18, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, O.; Schmidt, M.; Goebel, R.; Kuepper, H. Qualitative and quantitative three-dimensional accuracy of a single tooth captured by elastomeric impression materials: An in vitro study. J. Prosthet. Dent. 2012, 108, 165–172. [Google Scholar] [CrossRef]
- Soganci, G.; Cinar, D.; Caglar, A.; Yagiz, A. 3D evaluation of the effect of disinfectants on dimensional accuracy and stability of two elastomeric impression materials. Dent. Mater. J. 2018, 37, 675–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangano, F.; Gandolfi, A.; Luongo, G.; Logozzo, S. Intraoral scanners in dentistry: A review of the current literature. BMC Oral Health 2017, 17, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richert, R.; Goujat, A.; Venet, L.; Viguie, G.; Viennot, S.; Robinson, P.; Farges, J.C.; Fages, M.; Ducret, M. Intraoral scanner technologies: A review to make a successful impression. J. Healthc. Eng. 2017, 2017, 8427595. [Google Scholar] [CrossRef]
- Azar, B.; Eckert, S.; Kunkela, J.; Ingr, T.; Mounajjed, R. The marginal fit of lithium disilicate crowns: Press vs. CAD/CAM. Braz. Oral Res. 2018, 32, e001. [Google Scholar] [CrossRef] [Green Version]
- Sason, G.K.; Mistry, G.; Tabassum, R.; Shetty, O. A comparative evaluation of intraoral and extraoral digital impressions: An in vivo study. J. Indian Prosthodont. Soc. 2018, 18, 108. [Google Scholar] [CrossRef]
- Hazeveld, A.; Slater, J.J.; Ren, Y. Accuracy and reproducibility of dental replica models reconstructed by different rapid prototyping techniques. Am. J. Orthod. Dentofac. Orthop. 2014, 145, 108–115. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, K.B.; Kim, W.C.; Kim, J.H.; Kim, H.Y. Accuracy and precision of polyurethane dental arch models fabricated using a three-dimensional subtractive rapid prototyping method with an intraoral scanning technique. Korean J. Orthod. 2014, 44, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Papaspyridakos, P.; Chen, Y.W.; Alshawaf, B.; Kang, K.; Finkelman, M.; Chronopoulos, V.; Weber, H.P. Digital workflow: In vitro accuracy of 3D printed casts generated from complete-arch digital implant scans. J. Prosthet. Dent. 2020, 124, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Motel, C.; Kirchner, E.; Adler, W.; Wichmann, M.; Matta, R.E. Impact of Different Scan Bodies and Scan Strategies on the Accuracy of Digital Implant Impressions Assessed with an Intraoral Scanner: An In Vitro Study. J. Prosthodont. 2019, 29, 309–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO. ISO 5725-1: 1994, Accuracy (Trueness and Precision) of Measurement Methods and Results-Part 1: General Principles and Definitions; International Organization for Standardization: Geneva, Switzerland, 1994. [Google Scholar]
- Zimmermann, M.; Ender, A.; Mehl, A. Local accuracy of actual intraoral scanning systems for single-tooth preparations in vitro. J. Am. Dent. Assoc. 2019, 151, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Jeong, J.H.; Lee, J.I.; Cho, H.W. Effect of digital scans on marginal and internal discrepancies of zirconia crowns. J. Prosthet. Dent. 2019, 124, 461–467. [Google Scholar] [CrossRef]
- Nedelcu, R.; Olsson, P.; Nyström, I.; Thor, A. Finish line distinctness and accuracy in 7 intraoral scanners versus conventional impression: An in vitro descriptive comparison. BMC Oral Health 2018, 18, 27. [Google Scholar] [CrossRef]
- Mennito, A.S.; Evans, Z.P.; Nash, J.; Bocklet, C.; Lauer, A.; Bacro, T.; Cayouette, M.; Ludlow, M.; Renne, W.G. Evaluation of the trueness and precision of complete arch digital impressions on a human maxilla using seven different intraoral digital impression systems and a laboratory scanner. J. Esthet. Restor. Dent. 2019, 31, 369–377. [Google Scholar] [CrossRef]
- Mangano, F.G.; Admakin, O.; Bonacina, M.; Lerner, H.; Rutkunas, V.; Mangano, C. Trueness of 12 intraoral scanners in the full-arch implant impression: A comparative in vitro study. BMC Oral Health 2020, 20, 1–21. [Google Scholar] [CrossRef]
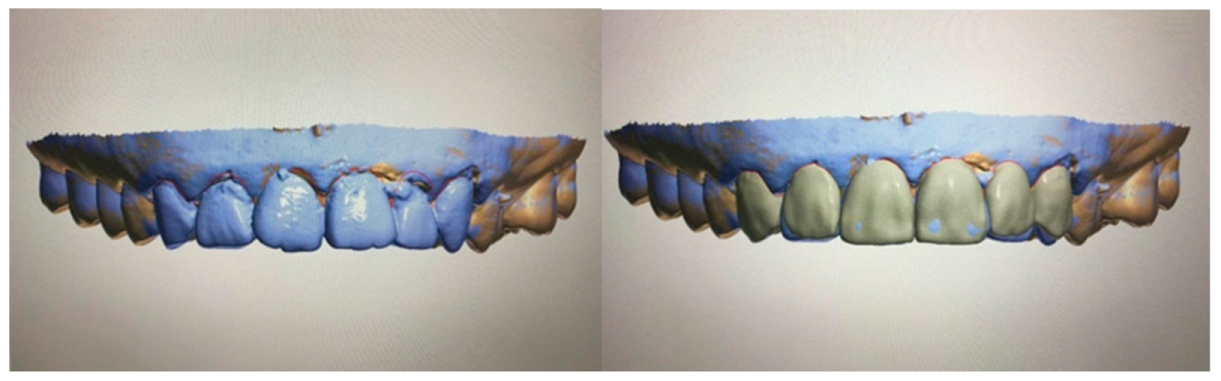

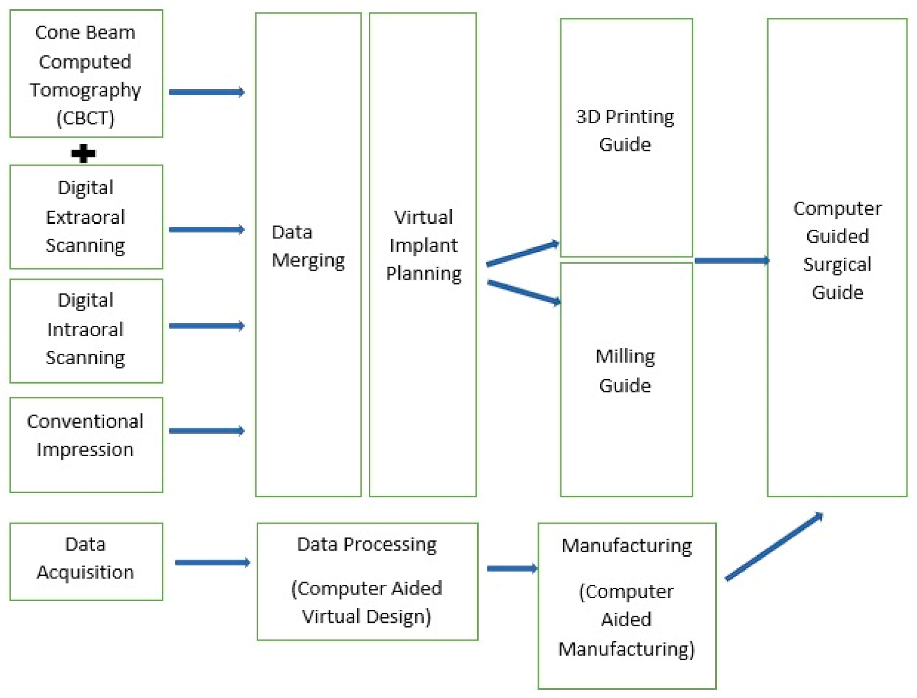

| Manufacturer | Software |
|---|---|
| 3 Shape | Implant Studio |
| Nobel Biocare | NobelClinician |
| Straumann | CoDiagnostix™ |
| Sirona | SICAT |
| Materialise | SimPlant® |
| Bredent | SKYplanX |
| 360Imaging | 360dps |
| BlueSky bio | BlueskyPlan |
| Anatomage | Anatomage guide |
| AstraTech dental | Facilitate |
| BioHorizons | VIP 3 |
| CyberMed | OnDemand3D™ |
| Swissmeda AG | Swissmeda Planning Solution |
| SICAT GmbH & Co. KG | SICAT Implant 2.0 |
| MIS | MGUIDE |
| Megagen Implant | R2 Gate |
| OSSTEM | OneGuide |
| Exocad | Implant Module |
| Amann Girrbach | Ceramill M-Plant (abutment module only) |
| Planmeca | Planmeca Romexis® 3D |
| Advantages | Disadvantages |
|---|---|
| Avoid risks of injuring important anatomical structures | Steep initial learning curve |
| Involve multidisciplinary approaches | High initial cost |
| Possible avoidance of complex bone regeneration/grafting technique | Increased preoperative surgical planning |
| Reduced surgical chairside time | Adequate mouth opening; challenges for microstomia patient or posterior implant placement. |
| Allow minimal surgical intervention (flapless surgery) | Limited visual on implant crestal depth location |
| Improve dentist-patient communication due to required preoperative planning | Risk of fracture on the surgical templates |
| Manufacturer | Material |
|---|---|
| NextDent | NexDent-SG |
| Stratasys | MED610 |
| EnvisionTec | E-Guide Tint |
| Formlabs | Surgical Guide Resin Dental SG Resin |
| Zortrax | Raydent Surgical Guide Resin |
| BEGO | VarseoWax Surgical Guide |
| SHERA | SHERAprint-sg |
| DentalMed | 3Delta Guide S |
| Carbon | Whip Mix Surgical Guide |
| Detax | FREEPRINT® splint 2.0 |
| 3D Systems | Visijet M3 Stoneplast |
| Zenith | ZMD-1000B CLEAR-SG |
| SprintRay | SprintRay Surgical Guide 2 |
| Shining 3D® | Resin Shining 3D Surgical Guide |
| Prodways Tech | PLASTCure Clear 200 |
| DMG | LuxaPrint Ortho |
| UNIZ | zSG (Surgical Guide) Resin |
| 3Dresyns | Dental 3Dresyns OD |
| Makex | Surgical Guide |
| VOCO | V-Print SG |
| Manufacturer | System |
|---|---|
| ClaroNav Technology Inc. | Navident |
| X-Nav Technologies | X Guide™ |
| Image Navigation | Image Guided Implant (IGI) Dentistry System |
| Neocis | YOMI® |
| Navigate Surgical | Inliant® |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alauddin, M.S.; Baharuddin, A.S.; Mohd Ghazali, M.I. The Modern and Digital Transformation of Oral Health Care: A Mini Review. Healthcare 2021, 9, 118. https://0-doi-org.brum.beds.ac.uk/10.3390/healthcare9020118
Alauddin MS, Baharuddin AS, Mohd Ghazali MI. The Modern and Digital Transformation of Oral Health Care: A Mini Review. Healthcare. 2021; 9(2):118. https://0-doi-org.brum.beds.ac.uk/10.3390/healthcare9020118
Chicago/Turabian StyleAlauddin, Muhammad Syafiq, Ahmad Syukran Baharuddin, and Mohd Ifwat Mohd Ghazali. 2021. "The Modern and Digital Transformation of Oral Health Care: A Mini Review" Healthcare 9, no. 2: 118. https://0-doi-org.brum.beds.ac.uk/10.3390/healthcare9020118






