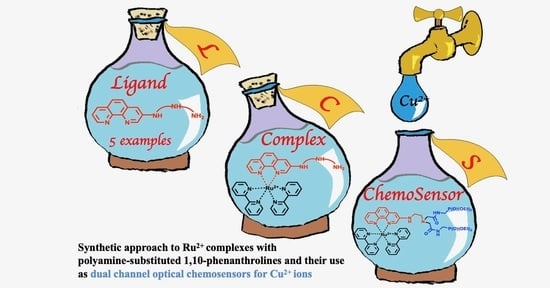
Ruthenium(II) Complexes with (3-Polyamino)phenanthrolines: Synthesis and Application in Sensing of Cu(II) Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus
2.3. Synthesis
2.4. Protonation and Complexation Studies
3. Results and Discussion
3.1. Synthesis
3.2. Optical Properties of Ru(N2phen) and Ru(N2P2phen)
3.3. Detection of Metal Cations in Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santos, J.L.; Farahi, F. (Eds.) Handbook of Optical Sensors; CRC Press: Boca Raton, FL, USA, 2014; 718p. [Google Scholar]
- Gunnlaugsson, T.; Leonard, J.P.; Murray, N.S. Highly selective colorimetric naked-eye Cu(II) detection using an Azobenzene Chemosensor. Org. Lett. 2004, 6, 1557–1560. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Kumar, S. A diamide–diamine based Cu2+ chromogenic sensor for highly selective visual and spectrophotometric detection. Tetrahedron Lett. 2006, 47, 4109–4112. [Google Scholar] [CrossRef]
- Ranyuk, E.; Douaihy, C.M.; Bessmertnykh, A.; Denat, F.; Averin, A.; Beletskaya, I.; Guilard, R. Diaminoanthraquinone-linked polyazamacrocycles: Efficient and simple colorimetric sensor for lead ion in aqueous solution. Org. Lett. 2009, 11, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-D.; Wolfbeis, O.S. Optical methods for sensing and imaging oxygen: Materials, spectroscopies and applications. Chem. Soc. Rev. 2014, 43, 3666–3761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Ye, Z.; Wang, G.; Zhang, W.; Yuan, J. Development of a ruthenium(II) complex based luminescent probe for imaging nitric oxide production in living cells. Chem. Eur. J. 2010, 16, 6884–6891. [Google Scholar] [CrossRef]
- Park, H.-J.; Chung, Y.K. Ru(II)–M(I) (M = Rh and Ir) bimetallic complexes based on a bridging ligand composed of 1,10-phenanthroline and N-heterocyclic carbene: Coordination chemistry and detection property of carbon monoxide. Inorg. Chim. Acta 2012, 391, 105–113. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, X.; Ye, Z.; Wang, G.; Zhang, W.; Yuan, J. Turn-on luminescent probe for cysteine/homocysteine based on a ruthenium(II) complex. Inorg. Chem. 2010, 49, 7898–7903. [Google Scholar] [CrossRef]
- Gill, M.R.; Thomas, J.A. Ruthenium(II) polypyridyl complexes and DNA—From structural probes to cellular imaging and therapeutics. Chem. Soc. Rev. 2012, 41, 3179–3192. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, F.; Wang, Y.-L.; Song, B.; Zhang, R.; Yuan, J. Red-emitting ruthenium(II) and iridium(III) complexes as phosphorescent probes for methylglyoxal in vitro and in vivo. Inorg. Chem. 2017, 56, 1309–1318. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, F.; Huang, C. Phosphorescent chemosensors based on heavy-metal complexes. Chem. Soc. Rev. 2010, 39, 3007–3030. [Google Scholar] [CrossRef]
- Mondal, D.; Bar, M.; Mukherjee, S.; Baitalik, S. Design of Ru(II) complexes based on anthraimidazoledione-functionalized terpyridine ligand for improvement of room-temperature luminescence characteristics and recognition of selective anions: Experimental and DFT/TD-DFT study. Inorg. Chem. 2016, 55, 9707–9724. [Google Scholar] [CrossRef]
- Padilla-Tosta, M.E.; Lloris, J.M.; Martinez-Máñez, R.; Benito, A.; Soto, J.; Pardo, T.; Miranda, M.A.; Marcos, M.D. Bis(terpyridyl)-ruthenium(II) units attached to polyazacycloalkanes as sensing fluorescent receptors for transition metal ions. Eur. J. Inorg. Chem. 2000, 2000, 741–748. [Google Scholar] [CrossRef]
- Watanabe, S.; Ikishima, S.; Matsuo, T.; Yoshida, K. A Luminescent metalloreceptor exhibiting remarkably high selectivity for Mg2+ over Ca2+. J. Am. Chem. Soc. 2001, 123, 8402–8403. [Google Scholar] [CrossRef]
- Yue, Y.; Huo, F.; Cheng, F.; Zhu, X.; Mafireyi, T.; Strongin, R.M.; Yin, C. Functional synthetic probes for selective targeting and multi-analyte detection and imaging. Chem. Soc. Rev. 2019, 48, 4155–4177. [Google Scholar] [CrossRef]
- Wang, J.-N.; Qi, Q.; Zhang, L.; Li, S.-H. Turn-on luminescent sensing of metal cations via quencher displacement: Rational design of a highly selective chemosensor for chromium(III). Inorg. Chem. 2012, 51, 13103–13107. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Mi, X.; Guan, L.; Tang, X.; Ju, Z.; Zhang, G.; Wang, C.; Liu, W. Design and application of a water-soluble phosphorescent Ru(II) complex as turn-on sensing material for Hg2+. J. Mater. Chem. B 2015, 3, 6205–6212. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Xiao, F.; Zhang, W.; Lin, C.; Wu, Y. Highly stable and NIR luminescent Ru–LPMSN hybrid materials for sensitive Detection of Cu2+ in vivo. ACS Appl. Mater. Interfaces 2018, 10, 26964–26971. [Google Scholar] [CrossRef]
- Kumar, S.; Arora, A.; Kaushal, J.; Oswal, P.; Kumar, A.; Kumar, P. Developing a simple and water soluble thiophene-functionalized Ru(II)-polypyridyl complex for ferric ion detection. Inorg. Chem. Commun. 2019, 107, 107500. [Google Scholar] [CrossRef]
- Wong, K.-L.; Bünzli, J.-C.G.; Tanner, P.A. Quantum yield and brightness. J. Lumin. 2020, 224, 117256. [Google Scholar] [CrossRef]
- Li, M.-J.; Chu, B.W.-K.; Zhu, N.; Yam, V.W.-W. Synthesis, structure, photophysics, electrochemistry, and ion-binding studies of ruthenium(II) 1,10-phenanthroline complexes containing thia-, selena-, and aza-crown pendants. Inorg. Chem. 2007, 46, 720–733. [Google Scholar] [CrossRef]
- Schmittel, M.; Lin, H.-W. Quadruple-channel sensing: A molecular sensor with a single type of receptor site for selective and quantitative multi-ion analysis. Angew. Chem. Int. Ed. 2007, 46, 893–896. [Google Scholar] [CrossRef] [PubMed]
- Curtright, A.E.; McCusker, J.K. Static and time-resolved spectroscopic studies of low-symmetry Ru(II) polypyridyl complexes. J. Phys. Chem. A 1999, 103, 7032–7041. [Google Scholar] [CrossRef] [Green Version]
- Abel, A.S.; Zenkov, I.S.; Averin, A.D.; Cheprakov, A.V.; Bessmertnykh-Lemeune, A.G.; Orlinson, B.S.; Beletskaya, I.P. Tuning the luminescent properties of ruthenium(II) amino-1,10-phenanthroline complexes by varying the position of the amino group on the heterocycle. ChemPlusChem 2019, 84, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Kálmán, F.K.; Woods, M.; Caravan, P.; Jurek, P.; Spiller, M.; Tircsó, G.; Király, R.; Brücher, E.; Sherry, A.D. Potentiometric and relaxometric properties of a gadolinium-based MRI contrast agent for sensing tissue pH. Inorg. Chem. 2007, 46, 5260–5270. [Google Scholar] [CrossRef] [Green Version]
- Ukai, T.; Kawazura, H.; Ishii, Y.; Bonnet, J.J.; Ibers, J.A. Chemistry of dibenzylideneacetone-palladium(0) complexes: I. Novel tris(dibenzylideneacetone)dipalladium(solvent) complexes and their reactions with quinones. J. Organomet. Chem. 1974, 65, 253–266. [Google Scholar] [CrossRef]
- Tzalis, D.; Tor, Y.; Salvatorre, F.; Jay Siegel, S. Simple one-step synthesis of 3-bromo- and 3,8-dibromo-1,10-phenanthroline: Fundamental building blocks in the design of metal chelates. Tetrahedron Lett. 1995, 36, 3489–3490. [Google Scholar] [CrossRef]
- Lay, P.A.; Sargeson, A.M.; Taube, H.; Chou, M.H.; Creutz, C. Cis-bis(2,2′-bipyridine-N,N′) complexes of ruthenium(III)/(II) and osmium(III)/(II). Inorg. Synth. 1986, 24, 291–299. [Google Scholar] [CrossRef]
- Albert, M.B. Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.J. Determination of the lower limit of detection. Clin. Chem. 1989, 35, 2152. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Beck, M.T.; Nagypál, I. Chemistry of Complex Equilibria; Ellis Horwood: Chichester, UK, 1990; 402p. [Google Scholar]
- Schmittel, M.; Ammon, H. Synthesis and spectroscopy of new iron(II) complexes of 4,7-bis(aza-crown ether)-phenanthrolines with unusual complexation properties. J. Chem. Soc. Chem. Commun. 1995, 687–688. [Google Scholar] [CrossRef]
- Schmittel, M.; Lin, H.-W.; Thiel, E.; Meixner, A.J.; Ammon, H. Effects of multiple ion loading on redox and luminescence properties of ruthenium trisphenanthroline crown ether hybrids. Dalton Trans. 2006, 4020–4028. [Google Scholar] [CrossRef]
- Suzuki, H.; Kanbara, T.; Yamamoto, T. Ru(II) complexes with new redox-active 1,10-phenanthroline derivatives: Structural, spectral, and electrochemical investigations. Inorg. Chim. Acta 2004, 357, 4335–4340. [Google Scholar] [CrossRef]
- Shan, G.-G.; Zhang, L.-Y.; Li, H.-B.; Wang, S.; Zhu, D.-X.; Li, P.; Wang, C.-G.; Su, Z.-M.; Liao, Y. A cationic iridium(III) complex showing aggregation-induced phosphorescent emission (AIPE) in the solid state: Synthesis, characterization and properties. Dalton Trans. 2012, 41, 523–530. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, T.; Zhan, L.; Zhong, C.; Gong, S.; Lu, Z.-H.; Yang, C. Tailoring optoelectronic properties of phenanthroline-based thermally activated delayed fluorescence emitters through isomer engineering. Adv. Opt. Mater. 2016, 4, 1558–1566. [Google Scholar] [CrossRef]
- Louis, M.; Thomas, H.; Gmelch, M.; Haft, A.; Fries, F.; Reineke, S. Blue-light-absorbing thin films showing ultralong room-temperature phosphorescence. Adv. Mater. 2019, 31, 1807887. [Google Scholar] [CrossRef]
- Liang, H.-P.; Acharjya, A.; Anito, D.A.; Vogl, S.; Wang, T.-X.; Thomas, A.; Han, B.-H. Rhenium-metalated polypyridine-based porous polycarbazoles for visible-light CO2 photoreduction. ACS Catal. 2019, 9, 3959–3968. [Google Scholar] [CrossRef]
- Wolfe, J.P.; Wagaw, S.; Marcoux, J.-F.; Buchwald, S.L. Rational development of practical catalysts for aromatic carbon−nitrogen bond formation. Acc. Chem. Res. 1998, 31, 805–818. [Google Scholar] [CrossRef]
- Hartwig, J.F. Carbon−heteroatom bond-forming reductive eliminations of amines, ethers, and sulfides. Acc. Chem. Res. 1998, 31, 852–860. [Google Scholar] [CrossRef]
- Ruiz-Castillo, P.; Buchwald, S.L. Applications of palladium-catalyzed C–N cross-coupling reactions. Chem. Rev. 2016, 116, 12564–12649. [Google Scholar] [CrossRef] [PubMed]
- Beletskaya, I.P.; Cheprakov, A.V. Copper in cross-coupling reactions. Coord. Chem. Rev. 2004, 248, 2337–2364. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Cheprakov, A.V. The complementary competitors: Palladium and copper in C–N cross-coupling reactions. Organometallics 2012, 31, 7753–7808. [Google Scholar] [CrossRef]
- Wagaw, S.; Buchwald, S.L. The synthesis of aminopyridines: A method employing palladium-catalyzed carbon−nitrogen bond formation. J. Org. Chem. 1996, 61, 7240–7241. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Shekhar, S.; Stambuli, J.P.; Hartwig, J.F. Highly Reactive, general, and long-lived catalysts for coupling heteroaryl and aryl chlorides with primary nitrogen nucleophiles. Angew. Chem. Int. Ed. 2005, 44, 1371–1375. [Google Scholar] [CrossRef]
- Eggert, J.P.W.; Lüning, U.; Näther, C. Synthesis and functionalisation of 5-substituted neocuproine derivatives. Eur. J. Org. Chem. 2005, 2005, 1107–1112. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Bessmertnykh, A.G.; Guilard, R. Palladium-catalyzed synthesis of aryl-substituted polyamine compounds from aryl halides. Tetrahedron Lett. 1997, 38, 2287–2290. [Google Scholar] [CrossRef]
- Cook, T.R.; Stang, P.J. Recent developments in the preparation and chemistry of metallacycles and metallacages via coordination. Chem. Rev. 2015, 115, 7001–7045. [Google Scholar] [CrossRef]
- Mede, T.; Jäger, M.; Schubert, U.S. “Chemistry-on-the-complex”: Functional RuII polypyridyl-type sensitizers as divergent building blocks. Chem. Soc. Rev. 2018, 47, 7577–7627. [Google Scholar] [CrossRef] [PubMed]
- Balzani, V.; Juris, A.; Venturi, M.; Campagna, S.; Serroni, S. Luminescent and redox-active polynuclear transition metal complexes. Chem. Rev. 1996, 96, 759–834. [Google Scholar] [CrossRef] [PubMed]
- Véry, T.; Ambrosek, D.; Otsuka, M.; Gourlaouen, C.; Assfeld, X.; Monari, A.; Daniel, C. Photophysical properties of ruthenium(II) polypyridyl DNA Intercalators: Effects of the molecular surroundings investigated by theory. Chem. Eur. J. 2014, 20, 12901–12909. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Ganguly, B.; Das, A. Urea-based ruthenium(II)−polypyridyl complex as an optical sensor for anions: Synthesis, characterization, and binding studies. Inorg. Chem. 2007, 46, 9912–9918. [Google Scholar] [CrossRef]
- Juris, A.; Balzani, V.; Barigelletti, F.; Campagna, S.; Belser, P.; von Zelewsky, A. Ru(II) polypyridine complexes: Photophysics, photochemistry, eletrochemistry, and chemiluminescence. Coord. Chem. Rev. 1988, 84, 85–277. [Google Scholar] [CrossRef]
- Cook, M.J.; Lewis, A.P.; McAuliffe, G.S.G.; Skarda, V.; Thomson, A.J.; Glasper, J.L.; Robbins, D.J. Luminescent metal complexes. Part 1. Tris-chelates of substituted 2,2′-bipyridyls with ruthenium (II) as dyes for luminescent solar collectors. J. Chem. Soc. Perkin Trans. 1984, 2, 1293–1301. [Google Scholar] [CrossRef]
- Vos, J.G. Excited-state acid-base properties of inorganic compounds. Polyhedron 1992, 11, 2285–2299. [Google Scholar] [CrossRef]
- Steinegger, A.; Wolfbeis, O.S.; Borisov, S.M. Optical sensing and imaging of pH values: Spectroscopies, materials, and applications. Chem. Rev. 2020, 120, 12357–12489. [Google Scholar] [CrossRef]
- Chan, C.-M.; Wong, K.-Y. Evaluation of a luminescent ruthenium complex immobilized inside Nafion as optical pH sensor. Analyst 1998, 123, 1843–1847. [Google Scholar] [CrossRef]
- Clarke, Y.; Xu, W.; Demas, J.N.; DeGraff, B.A. Lifetime-based pH sensor system based on a polymer-supported ruthenium(II) complex. Anal. Chem. 2000, 72, 3468–3475. [Google Scholar] [CrossRef]
- Malins, C.; Glever, H.G.; Keyes, T.E.; Vos, J.G.; Dressick, W.J.; MacCraith, B.D. Sol–gel immobilised ruthenium(II) polypyridyl complexes as chemical transducers for optical pH sensing. Sens. Actuators B 2000, 67, 89–95. [Google Scholar] [CrossRef]
- Hu, L.; Niu, C.; Zeng, G.; Wang, X.; Huang, D. Determination of dissolved oxygen in water based on its quenching effect on the fluorescent intensity of bis(2,2’-bipyridine)-5-amino-1,10-phenanthroline ruthenium complex. Anal. Sci. 2011, 27, 1121. [Google Scholar] [CrossRef] [Green Version]
- Giordano, P.J.; Bock, C.R.; Wrighton, M.S. Excited state proton transfer of ruthenium(II) complexes of 4,7-dihydroxy-1,10-phenanthroline. Increased acidity in the excited state. J. Am. Chem. Soc. 1978, 100, 6960–6965. [Google Scholar] [CrossRef]
- Thompson, A.M.W.C.; Smailes, M.C.C.; Jeffery, J.C.; Ward, M.D. Ruthenium tris-(bipyridyl) complexes with pendant protonatable and deprotonatable moieties: pH sensitivity of electronic spectral and luminescence properties. J. Chem. Soc. Dalton Trans. 1997, 737–744. [Google Scholar] [CrossRef]
- Granovsky, A.A. Firefly Version 8. Available online: http://classic.chem.msu.su/gran/firefly/index.html (accessed on 1 January 2022).
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.; et al. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Dolg, M.; Stoll, H.; Preuss, H.; Pitzer, R.M. Relativistic and correlation effects for element 105 (hahnium, Ha): A comparative study of M and MO (M = Nb, Ta, Ha) using energy-adjusted ab initio pseudopotentials. J. Phys. Chem. 1993, 97, 5852–5859. [Google Scholar] [CrossRef]
- Bryantsev, V.S.; Diallo, M.S.; Goddard, W.A. pKa calculations of aliphatic amines, diamines, and aminoamides via density functional theory with a Poisson−Boltzmann continuum solvent model. J. Phys. Chem. A 2007, 111, 4422–4430. [Google Scholar] [CrossRef] [Green Version]
- Abel, A.S.; Averin, A.D.; Cheprakov, A.V.; Roznyatovsky, V.A.; Denat, F.; Bessmertnykh-Lemeune, A.; Beletskaya, I.P. 6-Polyamino-substituted quinolines: Synthesis and multiple metal (CuII, HgII and ZnII) monitoring in aqueous media. Org. Biomol. Chem. 2019, 17, 4243–4260. [Google Scholar] [CrossRef] [PubMed]
- Ranyuk, E.; Uglov, A.; Meyer, M.; Bessmertnykh Lemeune, A.; Denat, F.; Averin, A.; Beletskaya, I.; Guilard, R. Rational design of aminoanthraquinones for colorimetric detection of heavy metal ions in aqueous solution. Dalton Trans. 2011, 40, 10491–10502. [Google Scholar] [CrossRef] [PubMed]
- Ballardini, R.; Varani, G.; Indelli, M.T.; Scandola, F.; Balzani, V. Free energy correlation of rate constants for electron transfer quenching of excited transition metal complexes. J. Am. Chem. Soc. 1978, 100, 7219–7223. [Google Scholar] [CrossRef]
- Ramdass, A.; Sathish, V.; Babu, E.; Velayudham, M.; Thanasekaran, P.; Rajagopal, S. Recent developments on optical and electrochemical sensing of copper(II) ion based on transition metal complexes. Coord. Chem. Rev. 2017, 343, 278–307. [Google Scholar] [CrossRef]
- Sivaraman, G.; Iniya, M.; Anand, T.; Kotla, N.G.; Sunnapu, O.; Singaravadivel, S.; Gulyani, A.; Chellappa, D. Chemically diverse small molecule fluorescent chemosensors for copper ion. Coord. Chem. Rev. 2018, 357, 50–104. [Google Scholar] [CrossRef]
- Okda, H.E.; El Sayed, S.; Ferreira, R.C.M.; Gonçalves, R.R.; Costa, S.P.G.; Raposo, M.M.M.; Martínez-Máñez, R.; Sancenón, F. N,N-Diphenylanilino-heterocyclic aldehyde-based chemosensors for UV-vis/NIR and fluorescence Cu(II) detection. New J. Chem. 2019, 43, 7393–7402. [Google Scholar] [CrossRef] [Green Version]
- Okda, H.E.; El Sayed, S.; Otri, I.; Ferreira, R.C.M.; Costa, S.P.G.; Raposo, M.M.M.; Martínez-Máñez, R.; Sancenón, F. 2,4,5-Triaryl imidazole probes for the selective chromo-fluorogenic detection of Cu(II). Prospective use of the Cu(II) complexes for the optical recognition of biothiols. Polyhedron 2019, 170, 388–394. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. Trace elements in human physiology and pathology. Copper. Biomed. Pharmacother. 2003, 57, 386–398. [Google Scholar] [CrossRef]
- Bisaglia, M.; Bubacco, L. Copper ions and Parkinson′s disease: Why is homeostasis so relevant? Biomolecules 2020, 10, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawle, S.C.; Moore, P.; Alcock, N.W. Synthesis and coordination chemistry of 1-(2′,2″-bipyridyl-5′-yl-methyl)-1,4,8,11-tetraazacyclotetradecane L1. Quenching of fluorescence from [Ru(bipy)2(L1)]2+ by coordination of NiII or CuII in the cyclam cavity (bipy = 2,2′-bipyridine; cyclam = 1,4,8,11-tetraazacyclotetradecane). J. Chem. Soc. Chem. Commun. 1992, 684–687. [Google Scholar] [CrossRef]
- Bolletta, F.; Costa, I.; Fabbrizzi, L.; Licchelli, M.; Montalti, M.; Pallavicini, P.; Prodi, L.; Zaccheroni, N. A [RuII(bipy)3]-[1,9-diamino-3,7-diazanonane-4,6-dione] two-component system, as an efficient ON–OFF luminescent chemosensor for Ni2+ and Cu2+ in water, based on an ET (energy transfer) mechanism. J. Chem. Soc. Dalton Trans. 1999, 1381–1386. [Google Scholar] [CrossRef]
- Website of ERA. Available online: https://www.epa.gov/eg/battery-manufacturing-effluent-guidelines-documents (accessed on 1 January 2022).
- Comba, P.; Krämer, R.; Mokhir, A.; Naing, K.; Schatz, E. Synthesis of new phenanthroline-based heteroditopic ligands—Highly efficient and selective fluorescence sensors for copper(II) ions. Eur. J. Inorg. Chem. 2006, 2006, 4442–4448. [Google Scholar] [CrossRef]
- Lin, Q.-T.; Pei, L.-M.; Xu, W.-C.; Chao, H.; Ji, L.-N. [Ru(bpy)2(pipdpa)]2+ as a highly sensitive and selective luminescent chemosensor for Cu2+ in aqueous solution. Inorg. Chem. Commun. 2012, 16, 104–106. [Google Scholar] [CrossRef]
- Zhang, P.; Pei, L.; Chen, Y.; Xu, W.; Lin, Q.; Wang, J.; Wu, J.; Shen, Y.; Ji, L.; Chao, H. A Dinuclear ruthenium(II) complex as a one- and two-photon luminescent probe for biological Cu2+ detection. Chem. Eur. J. 2013, 19, 15494–15503. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Tang, N.; Miao, K.; Wang, F. A dinuclear ruthenium(II) polypyridyl complex containing a terpy-like fragment for Cu2+ probing. Z. Anorg. Allg. Chem. 2014, 640, 1816–1821. [Google Scholar] [CrossRef]
- Zheng, Z.-B.; Kang, S.-Y.; Zhao, Y.; Zhang, N.; Yi, X.; Wang, K.-Z. pH and copper ion luminescence on/off sensing by a dipyrazinylpyridine-appended ruthenium complex. Sens. Actuators B 2015, 221, 614–624. [Google Scholar] [CrossRef]
- Liu, X.-W.; Xiao, Y.; Zhang, S.-B.; Lu, J.-L. A selective luminescent sensor for the detection of copper(II) ions based on a ruthenium complex containing DPA moiety. Inorg. Chem. Commun. 2017, 84, 56–58. [Google Scholar] [CrossRef]
- Zheng, Z.-B.; Huang, Q.-Y.; Han, Y.-F.; Zuo, J.; Ma, Y.-N. Ruthenium(II) complex-based chemosensors for highly sensitive and selective sequential recognition of copper ion and cyanide. Sens. Actuators B 2017, 253, 203–212. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, S. Copper ion luminescence on/off sensing by a quinoline-appended ruthenium(II)-polypyridyl complex in aqueous media. J. Mol. Struct. 2020, 1202, 127242. [Google Scholar] [CrossRef]
- Meyer, M.; Frémond, L.; Espinosa, E.; Guilard, R.; Ou, Z.; Kadish, K.M. Synthesis, characterization, and X-ray crystal structures of cyclam derivatives. 5. Copper(II) binding studies of a pyridine-strapped 5,12-dioxocyclam-based macrobicycle. Inorg. Chem. 2004, 43, 5572–5587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Z.; Zhang, Y.; Xu, Y.; Li, H.; Wang, C.; Lu, A.; Sun, S. A reversible and selective luminescent probe for Cu2+ detection based on a ruthenium(II) complex in aqueous solution. Sens. Actuators B 2015, 211, 449–455. [Google Scholar] [CrossRef]












 | |||||
|---|---|---|---|---|---|
| Entry | Ligand | (Pd)/Ligand (mol %) | Base | Yield, % 2 | |
| 3 | 4 | ||||
| 1 3 | - | - | Cs2CO3 4 | 0 5 | 0 |
| 2 3,6 | - | - | Cs2CO3 4 | 0 | 100 |
| 3 | BINAP 7 | 2/4 | tBuONa | traces | 100 |
| 4 | BINAP 7 | 2/25 | tBuONa | traces | 100 |
| 5 | BINAP 7 | 10/10.5 | tBuONa | 30 (28) | 70 |
| 6 | BINAP 7 | 10/10.5 | tBuONa | 30 | 70 |
| 7 | BINAP 7 | 10/25 | Cs2CO3 4 | <15 | 85 |
| 8 | BINAP 7 | 10/10.5 | K3PO4 4 | 0 | 100 |
| 9 8 | BINAP 7 | 10/10.5 | tBuONa | 16 | 80 |
| 10 | L1 | 10/10.5 | tBuONa | 35 | 60 |
| 11 | L2 | 10/10.5 | tBuONa | 0 | 100 |
| 12 | L3 | 10/10.5 | tBuONa | 35 | 65 |
| 13 | L4 | 10/10.5 | tBuONa | <15 | 85 |
| 14 | L5 | 10/10.5 | tBuONa | 60 (54) | 30 |
| 15 | L6 | 10/10.5 | tBuONa | 60 (50) | 30 |
| Complex | λabs, nm (log ε) 1 | λem, nm | Φ, % 2 | Brightness, B (λ = 450 nm), M−1 cm−1 3 |
|---|---|---|---|---|
| Ru(N2phen) | 285 (4.64) 350 (4.07) 450 (3.99) | 601 b | 4 | 396 |
| Ru(N2P2phen) | 285 (4.82) 350 (4.30) 450 (4.10) | 601 b | 3 | 415 |
| Entry | Compound | pKa | Reference |
|---|---|---|---|
| 1 | Ru(N2phen) | 9.13(7) | this work |
| 2 | 5a | 9.9–10.2 2 6.8–7.5 | [67] |
| 3 | Ru(N2P2phen) | 2.02(1) | this work |
| 4 | 9 | 2.26(1) | [68] |
| 5 | 10 | 2.11(1) | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abel, A.S.; Averin, A.D.; Cheprakov, A.V.; Beletskaya, I.P.; Meyer, M.; Bessmertnykh-Lemeune, A. Ruthenium(II) Complexes with (3-Polyamino)phenanthrolines: Synthesis and Application in Sensing of Cu(II) Ions. Chemosensors 2022, 10, 79. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors10020079
Abel AS, Averin AD, Cheprakov AV, Beletskaya IP, Meyer M, Bessmertnykh-Lemeune A. Ruthenium(II) Complexes with (3-Polyamino)phenanthrolines: Synthesis and Application in Sensing of Cu(II) Ions. Chemosensors. 2022; 10(2):79. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors10020079
Chicago/Turabian StyleAbel, Anton S., Alexei D. Averin, Andrey V. Cheprakov, Irina P. Beletskaya, Michel Meyer, and Alla Bessmertnykh-Lemeune. 2022. "Ruthenium(II) Complexes with (3-Polyamino)phenanthrolines: Synthesis and Application in Sensing of Cu(II) Ions" Chemosensors 10, no. 2: 79. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors10020079