Modeling the Dichromatic Behavior of Bromophenol Blue to Enhance the Analytical Performance of pH Colorimetric Sensor Arrays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instrumentation
2.2. Preparation of the CSA
2.3. Transduction of Color from “Analogic” to “Digital”
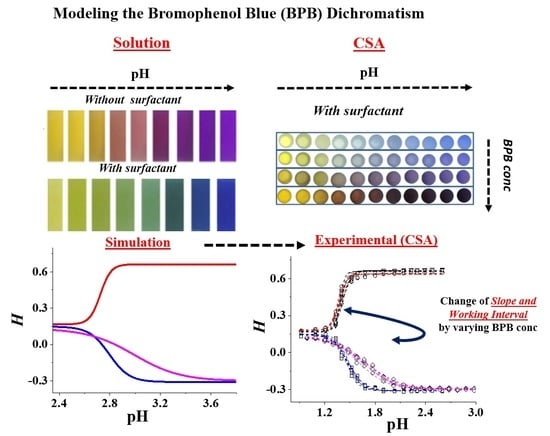
3. Results and Discussion
3.1. Absorption Spectra of the Solutions of BPB
3.2. Color Transduction from “Analogic” (Experimental) to “Digital” (s-RGB) for BPB Solutions
3.3. Simulated H Profiles in the Solution and Associated pH Prediction Error
3.4. Experimental H Profiles of the CSAs
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ko, Y.; Jeong, H.Y.; Kwon, G.; Kim, D.; Lee, C.; You, J. pH-responsive polyaniline/polyethylene glycol composite arrays for colorimetric sensor application. Sens. Actuators B Chem. 2020, 305, 127447. [Google Scholar] [CrossRef]
- Chen, H.; Ding, F.; Zhou, Z.; He, X.; Shen, J. FRET-based sensor for visualizing pH variation with colorimetric/ratiometric strategy and application for bioimaging in living cells, bacteria and zebrafish. Analyst 2020, 145, 4283–4294. [Google Scholar] [CrossRef] [PubMed]
- Capel-Cuevas, S.; Cuéllar, M.P.; de Orbe-Payá, I.; Pegalajar, M.C.; Capitán-Vallvey, L.F. Full-range optical pH sensor array based on neural networks. Microchem. J. 2011, 97, 225–233. [Google Scholar] [CrossRef]
- Caldara, M.; Colleoni, C.; Guido, E.; Re, V.; Rosace, G. Development of a textile-optoelectronic pH meter based on hybrid xerogel doped with Methyl Red. Sens. Actuators B Chem. 2012, 171–172, 1013–1021. [Google Scholar] [CrossRef]
- Martinez-Olmos, A.; Capel-Cuevas, S.; López-Ruiz, N.; Palma, A.J.; De Orbe, I.; Capitán-Vallvey, L.F. Sensor array-based optical portable instrument for determination of pH. Sens. Actuators B Chem. 2011, 156, 840–848. [Google Scholar] [CrossRef]
- Li, H.; Zhang, B.; Hu, W.; Liu, Y.; Dong, C.; Chen, Q. Monitoring black tea fermentation using a colorimetric sensor array-based artificial olfaction system. J. Food Processing Preserv. 2018, 42, e13348. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Suslick, K.S. Colorimetric Sensor Array for Monitoring CO and Ethylene. Anal. Chem. 2019, 91, 797–802. [Google Scholar] [CrossRef]
- Gotor, R.; Ashokkumar, P.; Hecht, M.; Keil, K.; Rurack, K. Optical pH Sensor Covering the Range from pH 0–14 Compatible with Mobile-Device Readout and Based on a Set of Rationally Designed Indicator Dyes. Anal. Chem. 2017, 89, 8437–8444. [Google Scholar] [CrossRef] [Green Version]
- Fairclough, S.M.; Giannetti, C.; Wagner, I.; Shakeel, H. Colorimetric sensor for pH monitoring of liquid samples using bubble wrap and mobile phone camera. In Proceedings of the 2020 IEEE International Conference on Flexible and Printable Sensors and Systems (FLEPS), Manchester, UK, 16–19 August 2020; pp. 2020–2023. [Google Scholar] [CrossRef]
- Kuswandi, B.; Asih, N.P.N.; Pratoko, D.K.; Kristiningrum, N.; Moradi, M. Edible pH sensor based on immobilized red cabbage anthocyanins into bacterial cellulose membrane for intelligent food packaging. Packag. Technol. Sci. 2020, 33, 321–332. [Google Scholar] [CrossRef]
- Huang, X.-w.; Zou, X.-b.; Shi, J.-y.; Li, Z.-h.; Zhao, J.-w. Colorimetric sensor arrays based on chemo-responsive dyes for food odor visualization. Trends Food Sci. Technol. 2018, 81, 90–107. [Google Scholar] [CrossRef]
- Ragain, J.C. A Review of Color Science in Dentistry: Colorimetry and Color Space. J. Dent. Oral Disord. Ther. 2016, 4, 1–5. [Google Scholar] [CrossRef]
- Pastore, A.; Badocco, D.; Pastore, P. High accuracy OrMoSil (Polyvinylidene Fluoride)-supported colorimetric sensor: Novel approach for the calculation of the pH prediction error. Talanta 2020, 213, 120840. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, A.A.; Mohamed, A.A. Determination of acid dissociation constants of Alizarin Red S, Methyl Orange, Bromothymol Blue and Bromophenol Blue using a digital camera. RSC Adv. 2020, 10, 11311–11316. [Google Scholar] [CrossRef] [Green Version]
- Cantrell, K.; Erenas, M.M.; De Orbe-Payá, I.; Capitán-Vallvey, L.F. Use of the hue parameter of the hue, saturation, value color space as a quantitative analytical parameter for bitonal optical sensors. Anal. Chem. 2010, 82, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.; Badocco, D.; Bogialli, S.; Cappellin, L.; Pastore, P. Ph colorimetric sensor arrays: Role of the color space adopted for the calculation of the prediction error. Sensors 2020, 20, 6036. [Google Scholar] [CrossRef] [PubMed]
- Pastore, A.; Badocco, D.; Pastore, P. Kinetic response of pH colorimetric sensors: Role of the cationic surfactant concentration and amount and type of solvent used in the preparation of the sensing spot. Microchem. J. 2020, 157, 104891. [Google Scholar] [CrossRef]
- Kreft, S.; Kreft, M. Quantification of dichromatism: A characteristic of color in transparent materials. J. Opt. Soc. Am. A 2009, 26, 1576. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.D. Quantifying Color Variation: Improved Formulas for Calculating Hue with Segment Classification. Appl. Plant Sci. 2014, 2, 1300088. [Google Scholar] [CrossRef]
- Kreft, S.; Kreft, M. Physicochemical and physiological basis of dichromatic colour. Naturwissenschaften 2007, 94, 935–939. [Google Scholar] [CrossRef]
- Huertas, R.; Yebra, A.; Pérez, M.M.; Melgosa, M.; Negueruela, A.I. Color Variability for a Wine Sample Poured into a Standard Glass Wine Sampler. Color Res. Appl. 2003, 28, 473–479. [Google Scholar] [CrossRef]
- Richer, S.P.; Little, A.C.; Adams, A.J. Effect of ophthalmic filter thickness on predicted monocular dichromatic luminance and chromaticity discrimination. Am. J. Optom. Physiol. Opt. 1984, 61, 666–673. [Google Scholar] [CrossRef]
- Sabnis, R.W. Handbook of Acid-Base Indicators; CRC Press: Boca Raton, FL, USA, 2007; pp. 1–416. [Google Scholar]
- Sun, W.; Li, H.; Wang, H.; Xiao, S.; Wang, J.; Feng, L. Sensitivity enhancement of pH indicator and its application in the evaluation of fish freshness. Talanta 2015, 143, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Suah, F.B.M.; Ahmad, M.; Taib, M.N. Applications of artificial neural network on signal processing of optical fibre pH sensor based on bromophenol blue doped with sol-gel film. Sens. Actuators B Chem. 2003, 90, 182–188. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, L. Preparation and optimization of optical pH sensor based on sol-gel. Sensors 2018, 18, 3195. [Google Scholar] [CrossRef] [Green Version]
- Marquardt, D.W. An Algorithm for Least-Squares Estimation of Nonlinear Parameters. J. Soc. Ind. Appl. Math. 2019, 11, 431–441. [Google Scholar] [CrossRef]
- Harris, A.C.; Weatherall, I.L. Objective evaluation of colour variation in the sandburrowing beetle chaerodes trachyscelides white (Coleoptera: Tenebrionidae) by instrumental determination of CIELAB values. J. R. Soc. N. Z. 1990, 20, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Otsu, H.; Yamamoto, M.; Hachisuka, T. Reproducing Spectral Reflectances From Tristimulus Colours. Comput. Graph. Forum 2018, 37, 370–381. [Google Scholar] [CrossRef]
- Lee, H.-C. Colorimetry. In Introduction to Color Imaging Science; Cambridge University Press: Cambridge, UK, 2010; pp. 89–131. [Google Scholar] [CrossRef]
- Rottman, C.; Grader, G.; De Hazan, Y.; Melchior, S.; Avnir, D. Surfactant-induced modification of dopants reactivity in sol-gel matrixes. J. Am. Chem. Soc. 1999, 121, 8533–8543. [Google Scholar] [CrossRef]
- Kodeh, F.S.; El-Nahhal, I.M.; Abd el-salam, F.H. Sol–Gel Encapsulation of Thymol Blue in Presence of Some Surfactants. Chem. Afr. 2019, 2, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Pastore, A.; Badocco, D.; Pastore, P. Reversible and high accuracy pH colorimetric sensor array based on a single acid-base indicator working in a wide pH interval. Talanta 2020, 219, 121251. [Google Scholar] [CrossRef]






| CT (M) | εmax(λ1) (Mcm)−1 | λ1 (nm) | εmax(λ2) (Mcm)−1 | λ2 (nm) | εmax(λ2)/εmax(λ1) | ∆λ (nm) |
|---|---|---|---|---|---|---|
| 0 | 24,157 | 437 | 69,637 | 590 | 2.6 | 153 |
| 0.0123 | 25,512 | 423 | 85,918 | 605 | 3.4 | 182 |
| Curve | Red | Black | Blue | Purple |
|---|---|---|---|---|
| CI (M) | 0.0064 | 0.0120 | 0.0230 | 0.0530 |
| −4.18 | −3.91 | −3.63 | −3.27 | |
| a | −11.35 | −10.47 | −6.11 | −4.23 |
| S | 8.29 | 7.44 | 4.16 | 2.52 |
| RSS | 0.309 | 0.795 | 0.408 | 0.472 |
| Adj. R-Square | 0.97 | 0.91 | 0.97 | 0.98 |
| pHi | 1.368 | 1.409 | 1.470 | 1.680 |
| ∆pH | 0.241 | 0.269 | 0.481 | 0.795 |
| 0.119 | 0.190 | 0.105 | 0.087 | |
| 0.014 | 0.026 | 0.025 | 0.035 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pastore, A.; Badocco, D.; Cappellin, L.; Pastore, P. Modeling the Dichromatic Behavior of Bromophenol Blue to Enhance the Analytical Performance of pH Colorimetric Sensor Arrays. Chemosensors 2022, 10, 87. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors10020087
Pastore A, Badocco D, Cappellin L, Pastore P. Modeling the Dichromatic Behavior of Bromophenol Blue to Enhance the Analytical Performance of pH Colorimetric Sensor Arrays. Chemosensors. 2022; 10(2):87. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors10020087
Chicago/Turabian StylePastore, Andrea, Denis Badocco, Luca Cappellin, and Paolo Pastore. 2022. "Modeling the Dichromatic Behavior of Bromophenol Blue to Enhance the Analytical Performance of pH Colorimetric Sensor Arrays" Chemosensors 10, no. 2: 87. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors10020087