Porous Pb-Doped ZnO Nanobelts with Enriched Oxygen Vacancies: Preparation and Their Chemiresistive Sensing Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemical Reagents
2.2. Preparation of Pb-Doped ZnO Porous Nanobelts
2.3. Fabrication and Measurements of Gas-Sensing Devices
2.4. Characterization
3. Results and Discussion
3.1. Preparation and Characterization of the Pb-Doped ZnO Porous Nanobelts
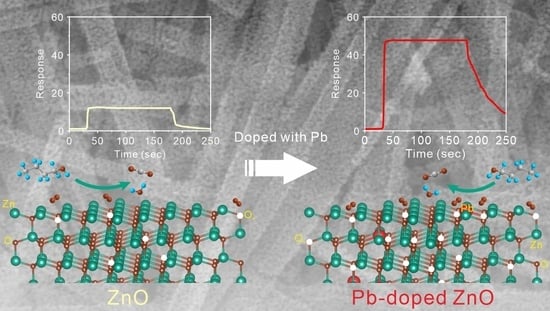
3.2. The Sensing Performance of Pb-Doped ZnO Porous Nanobelts
3.3. Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.; Zhang, W.; Yang, B.; Liu, J.; Chen, X.; Wang, X.; Yang, C. Gas-sensing enhancement methods for hydrothermal synthesized SnO2-based sensors. Nanotechnology 2017, 28, 452002. [Google Scholar] [CrossRef]
- Yuan, Z.; Han, E.; Meng, F.; Zuo, K. Detection and Identification of Volatile Organic Compounds Based on Temperature-Modulated ZnO Sensors. IEEE Trans. Instrum. Meas. 2019, 69, 4533–4544. [Google Scholar] [CrossRef]
- Zhao, Z.; Tian, J.; Sang, Y.; Cabot, A.; Liu, H. Structure, synthesis, and applications of TiO2 nanobelts. Adv. Mater. 2015, 27, 2557–2582. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Kim, J.S.; Lee, J.H. Rational Design of Semiconductor-Based Chemiresistors and their Libraries for Next-Generation Artificial Olfaction. Adv. Mater. 2020, 32, 2002075. [Google Scholar] [CrossRef]
- Bai, S.; Tian, K.; Han, N.; Guo, J.; Luo, R.; Li, D.; Chen, A. A novel rGO-decorated ZnO/BiVO4 heterojunction for the enhancement of NO2 sensing properties. Inorg. Chem. Front. 2020, 7, 1026–1033. [Google Scholar] [CrossRef]
- An, D.; Liu, N.; Zhang, H.; Sun, Q.; Li, C.; Li, Y.; Zhang, Q.; Lu, Y. Enhanced n-butanol sensing performance of SnO2-based gas sensors by doping In2O3 via co-precipitation method. Sens. Actuators B Chem. 2021, 340, 129944. [Google Scholar] [CrossRef]
- Nakayama, R.; Maesato, M.; Lim, G.; Arita, M.; Kitagawa, H. Heavy Hydrogen Doping into ZnO and the H/D Isotope Effect. J. Am. Chem. Soc. 2021, 143, 6616–6621. [Google Scholar] [CrossRef]
- Zunger, A.; Malyi, O.I. Understanding Doping of Quantum Materials. Chem. Rev. 2021, 121, 3031–3060. [Google Scholar] [CrossRef]
- Montazeri, A.; Jamali-Sheini, F. Enhanced ethanol gas-sensing performance of Pb-doped In2O3 nanostructures prepared by sonochemical method. Sens. Actuators B Chem. 2017, 242, 778–791. [Google Scholar] [CrossRef]
- Yousefi, R.; Zak, A.K.; Jamali-Sheini, F.; Huang, N.M.; Basirun, W.J.; Sookhakian, M. Synthesis and characterization of single crystal PbO nanoparticles in a gelatin medium. Ceram. Int. 2014, 40, 11699–11703. [Google Scholar] [CrossRef]
- Rao, A.; Long, H.; Harley-Trochimczyk, A.; Pham, T.; Zettl, A.; Carraro, C.; Maboudian, R. In Situ Localized Growth of Ordered Metal Oxide Hollow Sphere Array on Microheater Platform for Sensitive, Ultra-Fast Gas Sensing. ACS Appl. Mater. Interfaces 2017, 9, 2634–2641. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Zhang, B.; Li, Y.; Xu, X.; Sun, G.; Cao, J.; Wang, Y. Synthesis of spindle-like Co-doped LaFeO3 porous microstructure for high performance n-butanol sensor. Sens. Actuators B Chem. 2021, 343, 130125. [Google Scholar] [CrossRef]
- Guo, Z.; Li, M.-Q.; Liu, J.-H.; Huang, X.-J. Cation Exchange Synthesis and Unusual Resistive Switching Behaviors of Ag2Se Nanobelts. Small 2015, 11, 6285–6294. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Zheng, K.-G.; Wang, Z.; Chen, L.; Guo, Z. Cation-exchange synthesis of PbSe/ZnSe hetero-nanobelts with enhanced near-infrared photoelectronic performance. Nanotechnology 2021, 32, 335504. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.-B.; Li, Y.-X.; Su, Y.; Guo, Z.; Gu, C.-P.; Huang, J.-R.; Meng, F.-L.; Huang, X.-J.; Li, M.-Q.; Liu, J.-H. Porous and single-crystalline ZnO nanobelts: Fabrication with annealing precursor nanobelts, and gas-sensing and optoelectronic performance. Nanotechnology 2016, 27, 355702. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Fan, H.; Li, M.; Ma, L. Zeolitic Imidazolate Framework Coated ZnO Nanorods as Molecular Sieving to Improve Selectivity of Formaldehyde Gas Sensor. ACS Sens. 2015, 1, 243–250. [Google Scholar] [CrossRef]
- Pavithra, M.; Raj, M.J. Influence of ultrasonication time on solar light irradiated photocatalytic dye degradability and antibacterial activity of Pb doped ZnO nanocomposites. Ceram. Int. 2021, 47, 32324–32331. [Google Scholar] [CrossRef]
- Yang, D.; Su, X.; Yan, Y.; Hu, T.; Xie, H.; He, J.; Uher, C.; Kanatzidis, M.G.; Tang, X. Manipulating the Combustion Wave during Self-Propagating Synthesis for High Thermoelectric Performance of Layered Oxychalcogenide Bi1–xPbxCuSeO. Chem. Mater. 2016, 28, 4628–4640. [Google Scholar] [CrossRef]
- Li, X.B.; Ma, S.Y.; Li, F.M.; Yang, F.C.; Liu, J.; Zhang, X.L.; Wang, X. Blue-green and red luminescence from non-polar ZnO:Pb films. Appl. Surf. Sci. 2013, 270, 467–472. [Google Scholar] [CrossRef]
- Aboud, A.A.; Shaban, M.; Revaprasadu, N. Effect of Cu, Ni and Pb doping on the photo-electrochemical activity of ZnO thin films. RSC Adv. 2019, 9, 7729–7736. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; Liu, Z.; Yu, Y.; Zhang, C.; Chen, P.; Zhao, J.; He, P.; Guo, X. Synergistically Optimized Electron and Phonon Transport of Polycrystalline BiCuSeO via Pb and Yb Co-Doping. ACS Appl. Mater. Inter. 2021, 13, 57638–57645. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gu, D.; Li, X. Ultrasensitive NO2 Detection Utilizing Mesoporous ZnSe/ZnO Heterojunction-Based Chemiresistive-Type Sensors. ACS Appl. Mater. Interf. 2019, 11, 29029–29040. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Chen, X.; Huang, W.; Song, Y.; Li, J.; Chen, S.; Zhou, Y.; Dong, B.; Fan, D.; Zhu, X.; et al. Two-Dimensional Lead Monoxide: Facile Liquid Phase Exfoliation, Excellent Photoresponse Performance, and Theoretical Investigation. ACS Photon. 2018, 5, 5055–5067. [Google Scholar] [CrossRef]
- Kumar, P.; Liu, J.; Ranjan, P.; Hu, Y.; Yamijala, S.S.; Pati, S.K.; Cheng, G.J. Alpha Lead Oxide (alpha-PbO): A New 2D Material with Visible Light Sensitivity. Small 2018, 14, 1703346. [Google Scholar] [CrossRef]
- Liu, X.; Min, L.; Yu, X.; Zhou, Z.; Sha, L.; Zhang, S. Changes of photoelectrocatalytic, electrocatalytic and pollutant degradation properties during the growth of β-PbO2 into black titanium oxide nanoarrays. Chem. Eng. J. 2021, 417, 127996. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, S.; Liu, J.; Zheng, H.; Gu, Y.; Li, W.; Shi, H.; Li, S.; Zhong, X.; Wang, J. Lattice oxygen of PbO2 induces crystal facet dependent electrochemical ozone production. J. Mater. Chem. A 2021, 9, 9010–9017. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.; Wu, Z.; Dong, G. Metal-organic frameworks-derived hollow zinc oxide/cobalt oxide nanoheterostructure for highly sensitive acetone sensing. Sens. Actuators B Chem. 2018, 283, 42–51. [Google Scholar] [CrossRef]
- Jiang, W.; Low, J.; Mao, K.; Duan, D.; Chen, S.; Liu, W.; Pao, C.-W.; Ma, J.; Sang, S.; Shu, C. Pd-Modified ZnO-Au Enabling Alkoxy Intermediates Formation and Dehydrogenation for Photocatalytic Conversion of Methane to Ethylene. J. Am. Chem. Soc. 2021, 143, 269–278. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, S.; Fei, T.; Liu, S.; Zhang, T. Construction of ZnO/SnO2 Heterostructure on Reduced Graphene Oxide for Enhanced Nitrogen Dioxide Sensitive Performances at Room Temperature. ACS Sens. 2019, 4, 2048–2057. [Google Scholar] [CrossRef]
- Yan, W.; Xu, H.; Ling, M.; Zhou, S.; Qiu, T.; Deng, Y.; Zhao, Z.; Zhang, E. MOF-Derived Porous Hollow Co3O4@ZnO Cages for High-Performance MEMS Trimethylamine Sensors. ACS Sens. 2021, 6, 2613–2621. [Google Scholar] [CrossRef]
- Huang, B.; Zhang, Z.; Zhao, C.; Cairang, L.; Bai, J.; Zhang, Y.; Xie, E. Enhanced gas-sensing performance of ZnO@In2O3 core@shell nanofibers prepared by coaxial electrospinning. Sens. Actuators B Chem. 2018, 255, 2248–2257. [Google Scholar] [CrossRef]
- Liu, W.; Xu, L.; Sheng, K.; Chen, C.; Zhou, X.; Dong, B.; Bai, X.; Zhang, S.; Lu, G.; Song, H. APTES-functionalized thin-walled porous WO3 nanotubes for highly selective sensing of NO2 in a polluted environment. J. Mater. Chem. A 2018, 6, 10976–10989. [Google Scholar] [CrossRef]
- Li, L.; He, S.; Liu, M.; Zhang, C.; Chen, W. Three-Dimensional Mesoporous Graphene Aerogel-Supported SnO2 Nanocrystals for High-Performance NO2 Gas Sensing at Low Temperature. Anal. Chem. 2015, 87, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, K.; Zhou, H.; Fan, G.; Wang, Y.; Li, G.; Hu, R. A wireless-electrodeless quartz crystal microbalance with dissipation DMMP sensor. Sens. Actuators B Chem. 2018, 261, 408–417. [Google Scholar] [CrossRef]
- Koo, W.-T.; Choi, S.-J.; Kim, S.-J.; Jang, J.-S.; Tuller, H.L.; Kim, I.-D. Heterogeneous Sensitization of Metal–Organic Framework Driven Metal@Metal Oxide Complex Catalysts on an Oxide Nanofiber Scaffold Toward Superior Gas Sensors. J. Am. Chem. Soc. 2016, 138, 13431–13437. [Google Scholar] [CrossRef]
- Swaminathan, N.; Henning, A.; Jurca, T.; Hayon, J.; Shalev, G.; Rosenwaks, Y. Effect of varying chain length of n-alcohols and n-alkanes detected with electrostatically-formed nanowire sensor. Sens. Actuators B Chem. 2017, 248, 240–246. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Y.; Zhang, J.; Li, K.; Lv, T.; Shen, K.; Zhu, Z.; Liu, Q. Facile lotus-leaf-templated synthesis and enhanced xylene gas sensing properties of Ag-LaFeO3 nanoparticles. J. Mater. Chem. C 2018, 6, 6138–6145. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, Y.; Wang, L.; Lou, Z.; Qiao, L.; Tian, H.; Zheng, W. Ultrathin nanorod-assembled SnO2 hollow cubes for high sensitive n-butanol detection. Sens. Actuators B Chem. 2018, 283, 693–704. [Google Scholar] [CrossRef]
- Liu, F.; Huang, G.; Wang, X.; Xie, X.; Xu, G.; Lu, G.; He, X.; Tian, J.; Cui, H. High response and selectivity of single crystalline ZnO nanorods modified by In2O3 nanoparticles for n-butanol gas sensing. Sens. Actuators B Chem. 2019, 277, 144–151. [Google Scholar] [CrossRef]
- Yang, W.; Xiao, X.; Fang, B.; Deng, H. Nanorods-assembled ZnO microflower as a powerful channel for n-butanol sensing. J. Alloys Compd. 2021, 860, 158410. [Google Scholar] [CrossRef]
- Hoppe, M.; Ababii, N.; Postica, V.; Lupan, O.; Polonskyi, O.; Schütt, F.; Kaps, S.; Sukhodub, L.F.; Sontea, V.; Strunskus, T.; et al. (CuO-Cu2O)/ZnO:Al heterojunctions for volatile organic compound detection. Sens. Actuators B Chem. 2018, 255, 1362–1375. [Google Scholar] [CrossRef]
- Zhao, R.; Wei, Q.; Ran, Y.; Kong, Y.; Ma, D.; Su, L.; Yao, L.; Wang, Y. One-dimensional In2O3 nanorods as sensing material for ppb-level n-butanol detection. Nanotechnology 2021, 32, 375501. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ren, Y.; Guo, Y. ZrO2/ZnO nanocomposite materials for chemiresistive butanol sensors. Sens. Actuators B Chem. 2020, 308, 127658. [Google Scholar] [CrossRef]
- Wang, S.; Gao, X.; Yang, J.; Zhu, Z.; Zhang, H.; Wang, Y. Synthesis and gas sensor application of ZnFe2O4–ZnO composite hollow microspheres. RSC Adv. 2014, 4, 57967–57974. [Google Scholar] [CrossRef]
- Wang, M.; Shen, Z.; Zhao, X.; Duanmu, F.; Yu, H.; Ji, H. Rational shape control of porous Co3O4 assemblies derived from MOF and their structural effects on n-butanol sensing. J. Hazard. Mater. 2019, 371, 352–361. [Google Scholar] [CrossRef]
- Bai, J.; Li, Y.; Liu, Y.; Wang, H.; Liu, F.; Liu, F.; Sun, P.; Yan, X.; Lu, G. Au39Rh61 Alloy Nanocrystal-Decorated W18O49 for Enhanced Detection of n-Butanol. ACS Sens. 2019, 4, 2662–2670. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, L.; Yang, C.; Zheng, W.; Liu, X.; Zhang, J. Chemiresistive sensors based on core-shell ZnO@TiO2 nanorods designed by atomic layer deposition for n-butanol detection. Sens. Actuators B Chem. 2020, 310, 127846. [Google Scholar] [CrossRef]
- Lv, L.; Cheng, P.; Wang, Y.; Xu, L.; Zhang, B.; Lv, C.; Ma, J.; Zhang, Y. Sb-doped three-dimensional ZnFe2O4 macroporous spheres for N-butanol chemiresistive gas sensors. Sens. Actuators B Chem. 2020, 320, 128384. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, B.; Liu, J.; Yang, Q.; Cui, X.; Gao, Y.; Chuai, X.; Liu, F.; Sun, P.; Liang, X.; et al. Au-loaded mesoporous WO3: Preparation and n-butanol sensing performances. Sens. Actuators B Chem. 2016, 236, 67–76. [Google Scholar] [CrossRef]
- He, P.; Fu, H.; Yang, X.; Xiong, S.; Han, D.; An, X. Variable gas sensing performance towards different volatile organic compounds caused by integration types of ZnS on In2O3 hollow spheres. Sens. Actuators B Chem. 2021, 345, 130316. [Google Scholar] [CrossRef]
- Dang, F.; Wang, Y.; Gao, J.; Xu, L.; Cheng, P.; Lv, L.; Zhang, B.; Li, X.; Wang, C. Hierarchical flower-like NiCo2O4 applied in n-butanol detection at low temperature. Sens. Actuators B Chem. 2020, 320, 128577. [Google Scholar] [CrossRef]
- Cho, S.Y.; Yoo, H.W.; Kim, J.Y.; Jung, W.B.; Jin, M.L.; Kim, J.S.; Jeon, H.-J.; Jung, H.-T. High-Resolution p-Type Metal Oxide Semiconductor Nanowire Array as an Ultrasensitive Sensor for Volatile Organic Compounds. Nano Lett. 2016, 16, 4508–4515. [Google Scholar] [CrossRef] [PubMed]
- Shehada, N.; Brönstrup, G.; Funka, K.; Christiansen, S.; Leja, M.; Haick, H. Ultrasensitive Silicon Nanowire for Real-World Gas Sensing: Noninvasive Diagnosis of Cancer from Breath Volatolome. Nano Lett. 2014, 15, 1288–1295. [Google Scholar] [CrossRef]
- Alali, K.T.; Liu, J.; Liu, Q.; Li, R.; Zhang, H.; Aljebawi, K.; Liu, P.; Wang, J. Enhanced acetone gas sensing response of ZnO/ZnCo2O4 nanotubes synthesized by single capillary electrospinning technology. Sens. Actuators B Chem. 2017, 252, 511–522. [Google Scholar] [CrossRef]
- Wei, D.; Huang, Z.; Wang, L.; Chuai, X.; Zhang, S.; Lu, G. Hydrothermal synthesis of Ce-doped hierarchical flower-like In2O3 microspheres and their excellent gas-sensing properties. Sens. Actuators B Chem. 2018, 255, 1211–1219. [Google Scholar] [CrossRef]
- Spagnoli, E.; Krik, S.; Fabbri, B.; Valt, M.; Ardit, M.; Gaiardo, A.; Vanzetti, L.; Della Ciana, M.; Cristino, V.; Vola, G.; et al. Development and characterization of WO3 nanoflakes for selective ethanol sensing. Sens. Actuators B Chem. 2021, 347, 130593. [Google Scholar] [CrossRef]
- Masoumi, S.; Shokrani, M.; Aghili, S.; Hossein-Babaei, F. Zinc oxide-based direct thermoelectric gas sensor for the detection of volatile organic compounds in air. Sens. Actuators B Chem. 2019, 294, 245–252. [Google Scholar] [CrossRef]
- Postica, V.; Lupan, O.; Gapeeva, A.; Hansen, L.; Khaledialidusti, R.; Mishra, A.K.; Drewes, J.; Kersten, H.; Faupel, F.; Adelung, R.; et al. Improved Long-Term Stability and Reduced Humidity Effect in Gas Sensing: SiO2 Ultra-Thin Layered ZnO Columnar Films. Adv. Mater. Technol. 2021, 6, 2001137. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, K.-G.; Yang, T.-Y.; Guo, Z. Porous Pb-Doped ZnO Nanobelts with Enriched Oxygen Vacancies: Preparation and Their Chemiresistive Sensing Performance. Chemosensors 2022, 10, 96. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors10030096
Zheng K-G, Yang T-Y, Guo Z. Porous Pb-Doped ZnO Nanobelts with Enriched Oxygen Vacancies: Preparation and Their Chemiresistive Sensing Performance. Chemosensors. 2022; 10(3):96. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors10030096
Chicago/Turabian StyleZheng, Kai-Ge, Tian-Yu Yang, and Zheng Guo. 2022. "Porous Pb-Doped ZnO Nanobelts with Enriched Oxygen Vacancies: Preparation and Their Chemiresistive Sensing Performance" Chemosensors 10, no. 3: 96. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors10030096