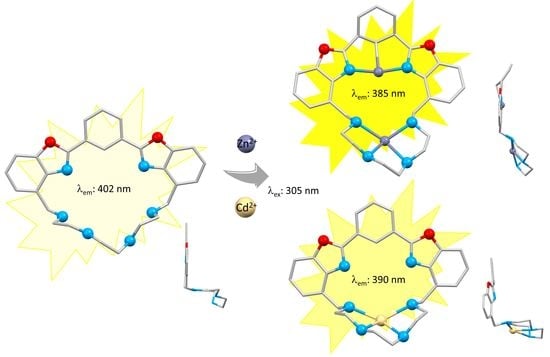
A New Benzoxazole-Based Fluorescent Macrocyclic Chemosensor for Optical Detection of Zn2+ and Cd2+
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Methods
2.2. Synthesis
2.2.1. 1,3-bis(4-Methyl-2-benzoxazolyl)phenyl (3)
2.2.2. 1,3-bis(4-Bromomethyl-2-benzoxazolyl)phenyl (4)
2.2.3. Hexacyclo[28.3.1.12,5.126,29.09,4.022,27]-35,36-dioxa-3,11,14,17,20,28-hexaaza-2,4,6,8,22,24,26,28,30,32,134-tetratricontaendecaene tetrahydrobromide (L·4HBr)
2.3. UV-Vis and Fluorescence Measurements
2.4. NMR Studies
2.5. Computational Analysis
3. Results and Discussion
3.1. Synthesis
3.2. Preliminary Screening
3.3. Acid–Base Behavior
3.3.1. UV-Vis Studies
3.3.2. 1H NMR Analysis
3.4. Metal Ion Complexation
3.4.1. UV-Vis Studies
3.4.2. NMR Studies
3.4.3. Computational Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, X.K.; Yeong, K.Y. A Patent Review on the Current Developments of Benzoxazoles in Drug Discovery. ChemMedChem 2021, 16, 3237–3262. [Google Scholar] [CrossRef] [PubMed]
- Arulmurugan, S.; Kavitha, H.P.; Vennila, J.P. Review on the Synthetic Methods of Biologically Potent Benzoxazole Derivatives. Mini. Rev. Org. Chem. 2020, 18, 769–785. [Google Scholar] [CrossRef]
- Temiz-Arpaci, O.; Zeyrek, C.T.; Arisoy, M.; Erol, M.; Celik, I.; Kaynak-Onurdag, F. Synthesis, Quantum Mechanical Calculations, Antimicrobial Activities and Molecular Docking Studies of Five Novel 2,5-Disubstituted Benzoxazole Derivatives. J. Mol. Struct. 2021, 1245, 131084. [Google Scholar] [CrossRef]
- Liu, H.; Xu, S.; Shi, X. Half-Sandwich Chromium(III) Complexes Containing Salicylbenzoxazole and Salicylbenzothiazole Ligands for Ethylene Polymerization. Inorg. Chem. Commun. 2021, 133, 108885. [Google Scholar] [CrossRef]
- Mao, S.; Han, X.; Li, C.; Huang, G.; Shen, K.; Shi, X.; Wu, H. Synthesis, Crystal Structure, Fluorescence and Electrochemical Properties of Two Ag(I) Complexes Based on 2-(4′-Pyridyl)-Benzoxazole/SPPh3 Ligands. J. Coord. Chem. 2018, 71, 3330–3341. [Google Scholar] [CrossRef]
- Chai, W.; Hong, M.; Song, L.; Jia, G.; Shi, H.; Guo, J.; Shu, K.; Guo, B.; Zhang, Y.; You, W.; et al. Three Reversible Polymorphic Copper(I) Complexes Triggered by Ligand Conformation: Insights into Polymorphic Crystal Habit and Luminescent Properties. Inorg. Chem. 2015, 54, 4200–4207. [Google Scholar] [CrossRef]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent Sensors for Measuring Metal Ions in Living Systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef]
- Gusev, A.N.; Shul’Gin, V.F.; Meshkova, S.B.; Smola, S.S.; Linert, W. A Novel Triazole-Based Fluorescent Chemosensor for Zinc Ions. J. Lumin. 2014, 155, 311–316. [Google Scholar] [CrossRef]
- Kumari, C.; Sain, D.; Kumar, A.; Debnath, S.; Saha, P.; Dey, S. Intracellular Detection of Hazardous Cd2+ through a Fluorescence Imaging Technique by Using a Nontoxic Coumarin Based Sensor. Dalt. Trans. 2017, 46, 2524–2531. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, X.; Zheng, M.; Yang, R.; Yang, H.; Jia, L.; Yang, M. Organic & Biomolecular Chemistry A 4,5-Quinolimide-Based Fluorescent Sensor for the Turn-on Detection of Cd2+ with Live-Cell Imaging. Org. Biomol. Chem. 2017, 15, 2211. [Google Scholar] [CrossRef]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Chemical Society Reviews CRITICAL REVIEW Fluorescent and Colorimetric Sensors for Detection of Lead, Cadmium, and Mercury Ions Fluorescent and Colorimetric Sensors for Detection of Lead, Cadmium, and Mercury Ions. Chem. Soc. Rev 2012, 41, 3210–3244. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, G.; Fanelli, M.; Paoli, P.; Formica, M.; Paderni, D.; Rossi, P.; Micheloni, M.; Giorgi, L.; Fusi, V. Zn(II) Detection and Biological Activity of a Macrocycle Containing a Bis(Oxadiazole)Pyridine Derivative as Fluorophore. Dalt. Trans. 2020, 49, 7496–7506. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, G.; Paz Clares, M.; Pont, I.; Formica, M.; Fusi, V.; Ricci, A.; Paoli, P.; Rossi, P.; García-España, E.; Inclán, M. Zn2+ and Cu2+ Complexes of a Fluorescent Scorpiand-Type Oxadiazole Azamacrocyclic Ligand: Crystal Structures, Solution Studies and Optical Properties. Dalt. Trans. 2020, 49, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Formica, M.; Ambrosi, G.; Fusi, V.; Giorgi, L.; Arca, M.; Garau, A.; Pintus, A.; Lippolis, V. CdII/ZnII Discrimination Using 2,5-Diphenyl[1,3,4]Oxadiazole Based Fluorescent Chemosensors. New J. Chem. 2018, 42, 7869–7883. [Google Scholar] [CrossRef]
- Formica, M.; Favi, G.; Fusi, V.; Giorgi, L.; Mantellini, F.; Micheloni, M. Synthesis and Study of Three Hydroxypyrazole-Based Ligands: A Ratiometric Fluorescent Sensor for Zn(II). J. Lumin. 2018, 195, 193–200. [Google Scholar] [CrossRef]
- Ambrosi, G.; Formica, M.; Fusi, V.; Giorgi, L.; Macedi, E.; Micheloni, M.; Paoli, P.; Rossi, P. A Biphenol-Based Chemosensor for ZnII and CdII Metal Ions: Synthesis, Potentiometric Studies, and Crystal Structures. Inorg. Chem. 2016, 55, 7676–7687. [Google Scholar] [CrossRef]
- Garau, A.; Lvova, L.; Macedi, E.; Ambrosi, G.; Aragoni, M.C.; Arca, M.; Caltagirone, C.; Coles, S.J.; Formica, M.; Fusi, V.; et al. N2S2 pyridinophane-Based Fluorescent Chemosensors for Selective Optical Detection of Cd2+ in Soils. New J. Chem. 2020, 44, 20834–20852. [Google Scholar] [CrossRef]
- Lvova, L.; Caroleo, F.; Garau, A.; Lippolis, V.; Giorgi, L.; Fusi, V.; Zaccheroni, N.; Lombardo, M.; Prodi, L.; Natale, C.D.; et al. A Fluorescent Sensor Array Based on Heteroatomic Macrocyclic Fluorophores for the Detection of Polluting Species in Natural Water Samples. Front. Chem. 2018, 6, 258. [Google Scholar] [CrossRef]
- Pathak, R.K.; Hinge, V.K.; Rai, A.; Panda, D.; Rao, C.P. Imino–Phenolic–Pyridyl Conjugates of Calix[4]Arene (L1 and L2) as Primary Fluorescence Switch-on Sensors for Zn2+ in Solution and in HeLa Cells and the Recognition of Pyrophosphate and ATP by [ZnL2]. Inorg. Chem. 2012, 51, 4994–5005. [Google Scholar] [CrossRef]
- Hagimori, M.; Uto, T.; Mizuyama, N.; Temma, T.; Yamaguchi, Y.; Tominaga, Y.; Saji, H. Fluorescence ON/OFF Switching Zn2+ Sensor Based on Pyridine–Pyridone Scaffold. Sens. Actuators B. Chem. 2013, 181, 823–828. [Google Scholar] [CrossRef]
- Mikata, Y.; Takekoshi, A.; Kaneda, M.; Konno, H.; Yasuda, K.; Aoyama, M.; Tamotsu, S. Replacement of Quinolines with Isoquinolines Affords Target Metal Ion Switching from Zn2+ to Cd2+ in the Fluorescent Sensor TQLN (N,N,N′,N′-Tetrakis(2-Quinolylmethyl)-2,6-Bis(Aminomethyl)Pyridine). Dalt. Trans. 2017, 46, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Suh, B.; Gil, D.; Yoon, S.; Kim, K.T.; Kim, C. A Practical Hydrazine-Carbothioamide-Based Fluorescent Probe for the Detection of Zn2+: Applications to Paper Strip, Zebrafish and Water Samples. Chemosensors 2022, 10, 32. [Google Scholar] [CrossRef]
- Ellairaja, S.; Manikandan, R.; Vijayan, M.T.; Rajagopal, S.; Vasantha, V.S. A Simple Highly Sensitive and Selective TURN-ON Fluorescent Chemosensor for the Detection of Cadmium Ions in Physiological Conditions. RSC Adv. 2015, 5, 63287–63295. [Google Scholar] [CrossRef]
- Goswami, S.; Aich, K.; Das, S.; Das Mukhopadhyay, C.; Sarkar, D.; Mondal, T.K. A New Visible-Light-Excitable ICT-CHEF-Mediated Fluorescence ‘Turn-on’ Probe for the Selective Detection of Cd2+ in a Mixed Aqueous System with Live-Cell Imaging. Dalt. Trans. 2015, 44, 5763–5770. [Google Scholar] [CrossRef]
- Kar, C.; Samanta, S.; Goswami, S.; Ramesh, A.; Das, G. A Single Probe to Sense Al(III) Colorimetrically and Cd(II) by Turn-on Fluorescence in Physiological Conditions and Live Cells, Corroborated by X-Ray Crystallographic and Theoretical Studies. Dalt. Trans. 2015, 44, 4123–4132. [Google Scholar] [CrossRef]
- Aich, K.; Goswami, S.; Das, S.; Das Mukhopadhyay, C.; Quah, C.K.; Fun, H.K. Cd2+ Triggered the FRET “ON”: A New Molecular Switch for the Ratiometric Detection of Cd2+ with Live-Cell Imaging and Bound X-Ray Structure. Inorg. Chem. 2015, 54, 7309–7315. [Google Scholar] [CrossRef]
- Vallee, B.L.; Falchuk, K.H. The Biochemical Basis of Zinc Physiology. Physiol. Rev. 1993, 73, 79–118. [Google Scholar] [CrossRef]
- Andreini, C.; Bertini, I.; Cavallaro, G. Minimal Functional Sites Allow a Classification of Zinc Sites in Proteins. PLoS ONE 2011, 6, e26325. [Google Scholar] [CrossRef] [Green Version]
- Nawrot, T.; Plusquin, M.; Hogervorst, J.; Roels, H.A.; Celis, H.; Thijs, L.; Vangronsveld, J.; Van Hecke, E.; Staessen, J.A. Environmental Exposure to Cadmium and Risk of Cancer: A Prospective Population-Based Study. Lancet Oncol. 2006, 7, 119–126. [Google Scholar] [CrossRef] [Green Version]
- Viau, M.; Collin-Faure, V.; Richaud, P.; Ravanat, J.L.; Candéias, S.M. Cadmium and T Cell Differentiation: Limited Impact in Vivo but Significant Toxicity in Fetal Thymus Organ Culture. Toxicol. Appl. Pharmacol. 2007, 223, 257–266. [Google Scholar] [CrossRef]
- Zalups, R.K.; Ahmad, S. Molecular Handling of Cadmium in Transporting Epithelia. Toxicol. Appl. Pharmacol. 2003, 186, 163–188. [Google Scholar] [CrossRef]
- Waisberg, M.; Joseph, P.; Hale, B.; Beyersmann, D. Molecular and Cellular Mechanisms of Cadmium Carcinogenesis. Toxicology 2003, 192, 95–117. [Google Scholar] [CrossRef]
- Satarug, S.; Baker, J.R.; Urbenjapol, S.; Haswell-Elkins, M.; Reilly, P.E.B.; Williams, D.J.; Moore, M.R. A Global Perspective on Cadmium Pollution and Toxicity in Non-Occupationally Exposed Population. Toxicol. Lett. 2003, 137, 65–83. [Google Scholar] [CrossRef]
- Bush, A.I. The Metallobiology of Alzheimer’s Disease. Trends Neurosci. 2003, 26, 207–214. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Koh, J.Y.; Bush, A.I. The Neurobiology of Zinc in Health and Disease. Nat. Rev. Neurosci. 2005, 6, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Zinc in Pancreatic Islet Biology, Insulin Sensitivity, and Diabetes. Prev. Nutr. Food Sci. 2017, 22, 1–8. [Google Scholar] [CrossRef]
- Ambrosi, G.; Battelli, C.; Formica, M.; Fusi, V.; Giorgi, L.; Macedi, E.; Micheloni, M.; Pontellini, R.; Prodi, L. Two Polyaminophenolic Fluorescent Chemosensors for H+ and Zn(II). Spectroscopic Behaviour of Free Ligands and of Their Dinuclear Zn(II) Complexes. New J. Chem. 2009, 33, 171–180. [Google Scholar] [CrossRef]
- Paderni, D.; Giorgi, L.; Macedi, E.; Formica, M.; Paoli, P.; Rossi, P.; Fusi, V. A Selective Fluorescent Probe for GadoliniumIII in Water Based on a PdII-Preorganized Chromone-Receptor. Dalt. Trans. 2021, 50, 15433. [Google Scholar] [CrossRef]
- Bradshaw, J.S.; Krakowiak, K.E.; Izatt, R.M. Aza-Crown Macrocycles; John Wiley & Sons Ltd.: New York, NY, USA, 1993. [Google Scholar]
- Weber, E. Crown Ethers and Analogs; Patai, S., Rappoport, Z., Eds.; John Wiley & Sons Ltd.: Chichester, NY, USA, 1989; ISBN 0471917079. [Google Scholar]
- Lindoy, L.F. The Chemistry of Macrocyclic Ligand Complexes; Cambridge University Press: Cambridge, MA, USA, 1989; ISBN 9780521252614. [Google Scholar]
- Chan, A.K.W.; Lam, E.S.H.; Tam, A.Y.Y.; Tsang, D.P.K.; Lam, W.H.; Chan, M.Y.; Wong, W.T.; Yam, V.W.W. Synthesis and Characterization of Luminescent Cyclometalated Platinum(II) Complexes of 1,3-Bis-Hetero-Azolylbenzenes with Tunable Color for Applications in Organic Light-Emitting Devices through Extension of π Conjugation by Variation of the Heteroatom. Chem. A Eur. J. 2013, 19, 13910–13924. [Google Scholar] [CrossRef]
- Ebara, K.; Shibasaki, Y.; Ueda, M. Photosensitive Poly(Benzoxazole) Based on Precursor from Diphenyl Isophthalate and Bis(o-Aminophenol). Polymer 2003, 44, 333–339. [Google Scholar] [CrossRef]
- Dang, T.T.; Soulé, J.F.; Doucet, H.; Benmensour, M.A.; Boucekkine, A.; Colombo, A.; Dragonetti, C.; Righetto, S.; Jacquemin, D.; Boixel, J.; et al. Asymmetrical 1,3-Bis(Heteroazolyl)Benzene Platinum Complexes with Tunable Second-Order Non-Linear Optical Properties. Eur. J. Inorg. Chem. 2016, 2016, 4774–4782. [Google Scholar] [CrossRef]
- Kuwabara, J.; Namekawa, T.; Sakabe, E.; Haga, M.a.; Kanbara, T. Luminescent Ir(III) Complexes Bearing Benzothiazole or Benzoxazole-Based Pincer Ligand. J. Organomet. Chem. 2017, 845, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.M.; Li, X.H.; Liang, C.; Mo, D.L. Base-Free Selective O-Arylation and Sequential [3,3]-Rearrangement of Amidoximes with Diaryliodonium Salts: Synthesis of 2-Substituted Benzoxazoles. Adv. Synth. Catal. 2017, 359, 4129–4135. [Google Scholar] [CrossRef]
- Bencini, A.; Bianchi, A.; Garcia-Espana, E.; Giusti, M.; Micheloni, M.; Paoletti, P. Solution Chemistry of Macrocycles. 5. Synthesis and Ligational Behavior toward Hydrogen and Copper(II) Ions of the Large Polyazacycloalkane 1,4,7,10,13,16,19,22,25-Nonaazacycloheptacosane ([27]AneN9). Inorg. Chem. 2002, 26, 681–684. [Google Scholar] [CrossRef]
- Mohanty, J.; Pal, H.; Sapre, A.V. Photophysical Properties of 2,2′- and 4,4′-Biphenyldiols. Bull. Chem. Soc. Jpn. 1999, 72, 2193–2202. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V.B. Methods for the Determination of Limit of Detection and Limit of Quantitation of the Analytical Methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-Functional Exchange-Energy Approximation with Correct Asymptotic Behavior. Phys. Rev. A 1988, 38, 3098. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P. Density-Functional Approximation for the Correlation Energy of the Inhomogeneous Electron Gas. Phys. Rev. B 1986, 33, 8822, Erratum in Phys. Rev. B 1986, 34, 7406. [Google Scholar] [CrossRef]
- Kim, K.; Jordan, K.D. Comparison of Density Functional and MP2 Calculations on the Water Monomer and Dimer. J. Phys. Chem. 2002, 98, 10089–10094. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 2002, 98, 11623–11627. [Google Scholar] [CrossRef]
- Cramer, C.J. Essentials of Computational Chemistry: Theories and Models, 2nd ed.; Wiley: Chichester, NH, USA, 2004; ISBN 978-0-470-09182-1. [Google Scholar]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully Optimized Contracted Gaussian Basis Sets of Triple Zeta Valence Quality for Atoms Li to Kr. J. Chem. Phys. 1998, 100, 5829. [Google Scholar] [CrossRef]
- Atkins, T.J.; Richman, J.E.; Oettle, W.F. Macrocyclic Polyamines: 1,4,7,10,13,16- Hesaazacyclooctadecane. Org. Synth. 1978, 58, 86. [Google Scholar] [CrossRef]
- Richman, J.E.; Atkins, T.J. Nitrogen Analogs of Crown Ethers. J. Am. Chem. Soc. 1974, 96, 2268–2270. [Google Scholar] [CrossRef]
- Czarnik, A.W. Fluorescent Chemosensors for Ion and Molecule Recognition; American Chemical Society: Washington, DC, USA, 1993. [Google Scholar]
- Amatori, S.; Ambrosi, G.; Errico Provenzano, A.; Fanelli, M.; Formica, M.; Fusi, V.; Giorgi, L.; Macedi, E.; Micheloni, M.; Paoli, P.; et al. PdII and PtII Complexes with a Thio-Aza Macrocycle Ligand Containing an Intercalating Fragment: Structural and Antitumor Activity Studies. J. Inorg. Biochem. 2016, 162, 154–161. [Google Scholar] [CrossRef]
- Ambrosi, G.; Formica, M.; Fusi, V.; Giorgi, L.; MacEdi, E.; Micheloni, M.; Paoli, P.; Pontellini, R.; Rossi, P. Efficient Fluorescent Sensors Based on 2,5-Diphenyl[1,3,4]Oxadiazole: A Case of Specific Response to Zn(II) at Physiological PH. Inorg. Chem. 2010, 49, 9940–9948. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, G.; Formica, M.; Fusi, V.; Giorgi, L.; Macedi, E.; Micheloni, M.; Piersanti, G.; Pontellini, R. New Family of Polyamine Macrocycles Containing 2,5-Diphenyl[1,3,4] Oxadiazole as a Signaling Unit. Synthesis, Acid-Base and Spectrophotometric Properties. Org. Biomol. Chem. 2010, 8, 1471–1478. [Google Scholar] [CrossRef]
- Ambrosi, G.; Formica, M.; Fusi, V.; Giorgi, L.; Macedi, E.; Micheloni, M.; Pontellini, R. A Family of Polyamino Phenolic Macrocyclic Ligands. Acid-Base and Coordination Properties towards Co(II), Ni(II), Cu(II), Zn(II), Cd(II) and Pb(II) Ions. Inorg. Chim. Acta 2009, 362, 3709–3714. [Google Scholar] [CrossRef]
- Paoli, P.; Rossi, P.; Ambrosi, G.; Formica, M.; Fusi, V.; Giorgi, L.; Micheloni, M.; Macedi, E. Structural Insights into a Versatile Macrocyclic Family Based on 2,5-Diphenyl[1,3,4]Oxadiazole: A Combined X-Ray Diffraction and Computational Study. Supramol. Chem. 2017, 29, 896–911. [Google Scholar] [CrossRef]
- Ambrosi, G.; Formica, M.; Fusi, V.; Giorgi, L.; Macedi, E.; Micheloni, M.; Paoli, P.; Rossi, P. Polynuclear Complexes: Two Amino-Phenol Macrocycles Spaced by Several Linear Polyamines; Synthesis, Binding Properties, and Crystal Structure. Inorg. Chem. 2009, 48, 10424–10434. [Google Scholar] [CrossRef] [Green Version]
- Brookhart, M.; Green, M.L.H.; Parkin, G. Agostic Interactions in Transition Metal Compounds. Proc. Natl. Acad. Sci. USA 2007, 104, 6908–6914. [Google Scholar] [CrossRef] [Green Version]
- Bonner, E.S.; Engle, J.T.; Sripothongak, S.; Ziegler, C.J. Zinc Complexes of the Carbahemiporphyrazines. Dalt. Trans. 2010, 39, 1932–1934. [Google Scholar] [CrossRef] [PubMed]











| Compound | ∆G (kcal/mol) 1 | ∆G (kcal/mol) 2 | DIST1 (Å) | DIST2 (Å) |
|---|---|---|---|---|
| [ZnL]2+ | −331.8 | −331.3 | 0.36 3 | 8.79 5 |
| [Zn2L]4+ | −367.5 | −366.4 | 0.33 3 | 8.84 5 |
| 0.072 4 | 6.20 6 | |||
| [CdL]2+ | −289.8 | −288.1 | 0.47 3 | 9.53 5 |
| [Cd2L]4+ | −277.1 | −275.2 | 0.49 3 | 9.57 5 |
| 0.23 4 | 6.79 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paderni, D.; Giorgi, L.; Voccia, M.; Formica, M.; Caporaso, L.; Macedi, E.; Fusi, V. A New Benzoxazole-Based Fluorescent Macrocyclic Chemosensor for Optical Detection of Zn2+ and Cd2+. Chemosensors 2022, 10, 188. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors10050188
Paderni D, Giorgi L, Voccia M, Formica M, Caporaso L, Macedi E, Fusi V. A New Benzoxazole-Based Fluorescent Macrocyclic Chemosensor for Optical Detection of Zn2+ and Cd2+. Chemosensors. 2022; 10(5):188. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors10050188
Chicago/Turabian StylePaderni, Daniele, Luca Giorgi, Maria Voccia, Mauro Formica, Lucia Caporaso, Eleonora Macedi, and Vieri Fusi. 2022. "A New Benzoxazole-Based Fluorescent Macrocyclic Chemosensor for Optical Detection of Zn2+ and Cd2+" Chemosensors 10, no. 5: 188. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors10050188