Recent Progresses in NIR-II Luminescent Bio/Chemo Sensors Based on Lanthanide Nanocrystals
Abstract
:1. Introduction
2. Design and Synthesis of NIR-II LnNCs
2.1. Mechanisms of NIR-II Luminescence

2.2. Strategies of Optimizing NIR-II Luminescence
2.2.1. Ion Doping
2.2.2. Core-Shell Structure
2.2.3. Dye-Sensitized Luminescence
2.3. Preparation Methods
2.3.1. Thermal Decomposition
2.3.2. Hydrothermal Synthesis
2.3.3. Co-Precipitation
3. Sensing Mechanisms of NIR-II LnNCs
3.1. Inorganic-Ion-Based Recognition Mechanism
3.2. Organic-Dye-Based Recognition Mechanism
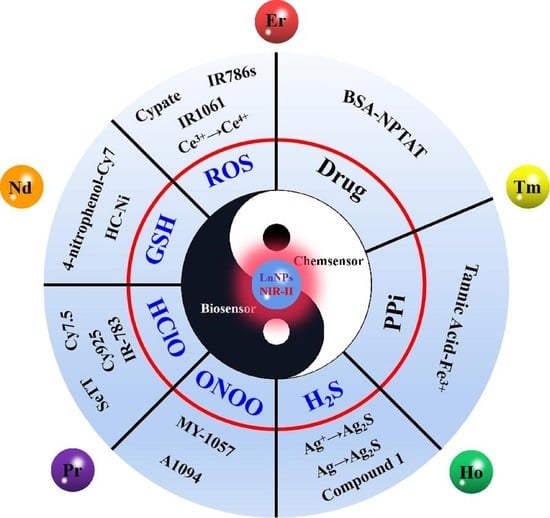
4. Category of Sensing
4.1. Biomedical Related Species
4.1.1. Reactive Oxygen Species (ROS)
4.1.2. HClO
4.1.3. Glutathione (GSH)
4.1.4. ONOO−
4.1.5. H2S
4.2. Chemical Species
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Li, Y.; Koo, S.; Sun, Y.; Liu, Y.; Liu, X.; Pan, Y.; Zhang, Z.; Du, M.; Lu, S.; et al. Versatile types of inorganic/organic NIR-IIa/IIb fluorophores: From strategic design toward molecular imaging and theranostics. Chem. Rev. 2022, 122, 209–268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.T.; Wang, Y.H.; Zhang, H.J. Lanthanide-doped fluorescence probes for NIR-II fluorescence imaging. Chin. J. Lumin. 2020, 41, 1460–1478. [Google Scholar] [CrossRef]
- Bhat, M.P.; Kurkuri, M.; Losic, D.; Kigga, M.; Altalhi, T. New optofluidic based lab-on-a-chip device for the real-time fluoride analysis. Anal. Chim. Acta 2021, 1159, 338439. [Google Scholar] [CrossRef] [PubMed]
- Purves, R.W.; Souster, K.; West, M.; Huda, A.M.; Fisher, C.M.E.; Belford, M.W.; Shurmer, B.O. Improved thyreostatic drug detection in animal tissues using liquid chromatography-high-field asymmetric waveform ion mobility spectrometry-mass spectrometry. J. Agric. Food Chem. 2022, 70, 4785–4791. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Electrochemical detection for microscale analytical systems: A review. Talanta 2002, 56, 223–231. [Google Scholar] [CrossRef]
- An, X.; Zhu, X.; Liu, J.; Zou, L.; Li, G.; Ye, B. Ratiometric fluorescence detection of ciprofloxacin using the terbium-based coordination polymers. Spectrochim. Acta A 2022, 269, 120775. [Google Scholar] [CrossRef]
- Liu, S.; Yan, L.; Huang, J.; Zhang, J.; Zhou, B. Controlling upconversion in emerging multilayer core-shell nanostructures: From fundamentals to frontier applications. Chem. Soc. Rev. 2022, 51, 1729–1765. [Google Scholar] [CrossRef]
- Cai, L.; Wang, Z.; Lin, B.; Liu, K.; Wang, Y.; Yuan, Y.; Tao, X.; Lv, R. Rare earth nanoparticles for sprayed and intravenous NIR II imaging and photodynamic therapy of tongue cancer. Nanoscale Adv. 2022, 4, 2224–2232. [Google Scholar] [CrossRef]
- Tao, W.; Farokhzad, O.C. Theranostic nanomedicine in the NIR-II window: Classification, fabrication, and biomedical applications. Chem. Rev. 2022, 122, 5405–5407. [Google Scholar] [CrossRef]
- Liang, T.; Guo, Z.; He, Y.; Wang, Y.; Li, C.; Li, Z.; Liu, Z. Cyanine-doped lanthanide metal-organic frameworks for near-Infrared II bioimaging. Adv. Sci. 2022, 9, 2104561. [Google Scholar] [CrossRef]
- Zhao, M.Y.; Li, B.H.; Zhang, H.X.; Zhang, F. Activatable fluorescence sensors for in vivo bio-detection in the second near-infrared window. Chem. Sci. 2021, 12, 3448. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.Q.; Wei, R.Y.; Sun, L.N. Lanthanide nanoparticles with efficient near-infrared-II emission for biological applications. J. Mater. Chem. B 2020, 8, 10257. [Google Scholar] [CrossRef] [PubMed]
- Diao, S.; Hong, G.S.; Antaris, A.L.; Blackburn, J.L.; Cheng, K.; Cheng, Z.; Dai, H.J. Biological imaging without autofluorescence in the second near-infrared region. Nano Res. 2015, 8, 3027–3034. [Google Scholar] [CrossRef]
- Hong, G.S.; Antaris, A.L.; Dai, H.J. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1, 0010. [Google Scholar] [CrossRef]
- Naczynski, D.J.; Tan, M.C.; Zevon, M.; Wall, B.; Kohl, J.; Kulesa, A.; Chen, S.; Roth, C.M.; Riman, R.E.; Moghe, P.V. Rare-earth-doped biological composites as in vivo shortwave infrared reporters. Nat. Commun. 2013, 4, 2199. [Google Scholar] [CrossRef]
- Tsukasaki, Y.; Komatsuzaki, A.; Mori, Y.; Ma, Q.; Yoshioka, Y.; Jin, T. A short-wavelength infrared emitting multimodal probe for non-invasive visualization of phagocyte cell migration in living mice. Chem. Commun. 2014, 50, 14356–14359. [Google Scholar] [CrossRef]
- Lei, Z.H.; Zhang, F. Molecular engineering of NIR-II fluorophores for improved biomedical detection. Angew. Chem. Int. Ed. 2021, 133, 16430–16444. [Google Scholar] [CrossRef]
- Zhong, Y.; Dai, H.J. A mini-review on rare-earth down-conversion nanoparticles for NIR-II imaging of biological systems. Nano Res. 2020, 13, 1281–1294. [Google Scholar] [CrossRef]
- Tang, Y.F.; Pei, F.; Lu, X.M.; Fan, Q.L.; Huang, W. Recent advances on activatable NIR-II fluorescence probes for biomedical imaging. Adv. Opt. Mater. 2019, 7, 1900917. [Google Scholar] [CrossRef]
- Kong, Y.; Chen, J.; Fang, H.; Heath, G.; Wo, Y.; Wang, W.; Li, Y.; Guo, Y.; Evans, S.D.; Chen, S.; et al. Highly fluorescent ribonuclease-A-encapsulated lead sulfide quantum dots for ultrasensitive fluorescence in vivo imaging in the second near-infrared window. Chem. Mater. 2016, 28, 3041–3050. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Tang, Y.; Ling Liu, L.; Lv, X.Y.; Wang, Q.L.; Ke, H.; Deng, Y.B.; Yang, H.; Yang, X.; Liu, G.; et al. Size-dependent Ag2S nanodots for second near-infrared fluorescence/photoacoustics imaging and simultaneous photothermal therapy. ACS Nano 2017, 11, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Hong, G.; Liang, Y.; Zhang, B.; Yaghi, O.K.; Dai, H.J. In vivo fluorescence imaging in the second near-infrared window with long circulating carbon nanotubes capable of ultrahigh tumor uptake. J. Am. Chem. Soc. 2012, 134, 10664–10669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, H.; Yue, J.; Zhu, S.; Uno, T.; Zhang, X.; Yang, Q.; Yu, K.; Hong, G.; Wang, J.; Li, L.; et al. A bright organic NIR-II nanofluorophore for three-dimensional imaging into biological tissues. Nat. Commun. 2018, 9, 1171. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.P.; Yan, L.F. Organic fluorescent nanoparticles with NIR-II emission for bioimaging and therapy. Biomed. Mater. 2021, 16, 022001. [Google Scholar] [CrossRef]
- Wang, S.F.; Li, B.H.; Zhang, F. Molecular fluorophores for deep-tissue bioimaging. ACS Cent. Sci. 2020, 6, 1302–1316. [Google Scholar] [CrossRef]
- Sun, W.; Guo, S.; Hu, C.; Fan, J.; Peng, X. Recent Development of chemosensors based on cyanine platforms. Chem. Rev. 2016, 116, 7768–7817. [Google Scholar] [CrossRef]
- Wanderi, K.; Cui, Z.Q. Organic fluorescent nanoprobes with NIR-IIb characteristics for deep learning. Exploration 2022, 2, 20210097. [Google Scholar] [CrossRef]
- Zhou, H.J.; Ren, T.B. Recent progress of cyanine fluorophores for NIR-II sensing and imaging. Chem. Asian J. 2022, 17, e202200147. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Ma, H.; Wang, H.; Zhang, R.; Zhang, X.D. Activatable NIR-II organic fluorescent probes for bioimaging. Theranostics 2022, 12, 3345–3371. [Google Scholar] [CrossRef]
- Yu, Z.; He, Y.; Schomann, T.; Wu, K.; Hao, Y.; Suidgeest, E.; Zhang, H.; Eich, C.; Cruz, L.J. Achieving effective multimodal imaging with rare-earth ion-doped CaF2 nanoparticles. Pharmaceutics 2022, 14, 840. [Google Scholar] [CrossRef]
- Xu, H.; Yang, Y.; Lu, L.; Yang, Y.; Zhang, Z.; Zhao, C.X.; Zhang, F.; Fan, Y. Orthogonal multiplexed NIR-II imaging with excitation-selective lanthanide-based nanoparticles. Anal. Chem. 2022, 94, 3661–3668. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.X.; Yang, Y.F.; Yang, X.F.; Pan, Y.; Sun, L.D.; Zhang, W.Y.; Shao, Y.; Shen, J.; Lin, J.; Li, L.; et al. Upconverted/downshifted NaLnF4 and metal-organic framework heterostructures boosting NIR-II imaging-guided photodynamic immunotherapy toward tumors. Nano Today 2022, 43, 101439. [Google Scholar] [CrossRef]
- Lv, Z.; Jin, L.; Cao, Y.; Zhang, H.; Xue, D.; Yin, N.; Zhang, T.; Wang, Y.; Liu, J.; Liu, X.; et al. A nanotheranostic agent based on Nd3+-doped YVO4 with blood-brain-barrier permeability for NIR-II fluorescence imaging/magnetic resonance imaging and boosted sonodynamic therapy of orthotopic glioma. Light Sci Appl 2022, 11, 116. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Guo, Z.; Yuan, W.; Kong, M.; Liu, Y.; Liu, Y.T.; Gao, Y.; Feng, W.; Wang, F.; Zhou, J.; et al. High-sensitivity imaging of time-domain near-infrared light transducer. Nat. Photonics 2019, 13, 525–531. [Google Scholar] [CrossRef]
- Ding, S.W.; Lu, L.F.; Fan, Y.; Zhang, F. Recent progress in NIR-Ⅱ emitting lanthanide-based nanoparticles and their biological applications. J. Rare Earths 2020, 38, 451–463. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, F. A new generation of NIR-Ⅱ probes: Lanthanide-based nanocrystals for bioimaging and biosensing. Adv. Opt. Mater. 2019, 7, 1801417. [Google Scholar] [CrossRef]
- Wang, Z.; Xing, B.G. Near-infrared multipurpose lanthanide-imaging nanoprobes. Chem.-Asian J. 2020, 15, 2076–2091. [Google Scholar] [CrossRef]
- Xu, J.; Gulzar, A.; Yang, P.P.; Bi, H.; Yang, D.; Gai, S.; He, F.; Lin, J.; Xing, B.G.; Jin, D.Y. Recent advances in near-infrared emitting lanthanide-doped nanoconstructs: Mechanism, design and application for bioimaging. Coord. Chem. Rev. 2019, 381, 104–134. [Google Scholar] [CrossRef]
- Yang, Y.J.; Tu, D.; Zhang, Y.; Zhang, P.; Chen, X.Y. Recent advances in design of lanthanide-containing NIR-II luminescent nanoprobes. iScience 2021, 24, 102062. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Zhai, X.Y.; Sun, M.Z.; Ma, T.F.; Huang, Y.K.; Huang, B.L.; Du, Y.P.; Yan, C.H. When rare earth meets carbon nanodots: Mechanisms, applications and outlook. Chem. Soc. Rev. 2020, 49, 9220–9248. [Google Scholar] [CrossRef]
- Yu, S.H.; Tu, D.; Lian, W.; Xu, J.; Chen, X.Y. Lanthanide-doped near-infrared II luminescent nanoprobes for bioapplications. Sci. China Mater. 2019, 62, 1071–1086. [Google Scholar] [CrossRef] [Green Version]
- Lia, Z.; Ding, X.; Cong, H.; Wang, S.; Yu, B.; Shen, Y. Recent advances on inorganic lanthanide-doped NIR-II fluorescence nanoprobes for bioapplication. J. Lumin. 2020, 228, 117627. [Google Scholar] [CrossRef]
- Zhang, H.X.; Fan, Y.; Pei, P.; Sun, C.; Lu, L.; Zhang, F. Tm3+-sensitized NIR-II fluorescent nanocrystals for in vivo information storage and decoding. Angew. Chem. Int. Ed. 2019, 58, 10153–10157. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, S.F.; Zhao, B.Z.; Pei, P.; Fan, Y.; Li, X.M.; Zhang, F. Er3+ sensitized 1530 nm to 1180 nm second near-infrared window upconversion nanocrystals for in vivo biosensing. Angew. Chem. Int. Ed. 2018, 57, 7518–7522. [Google Scholar] [CrossRef]
- Li, Y.B.; Zeng, S.J.; Hao, J.H. Non-invasive optical guided tumor metastasis/vessel imaging by using lanthanide nanoprobe with enhanced down-shifting emission beyond 1500 nm. ACS Nano 2019, 13, 248–259. [Google Scholar] [CrossRef]
- Zhong, Y.; Ma, Z.; Zhu, S.; Yue, J.; Zhang, M.; Antaris, A.L.; Yuan, J.; Cui, R.; Wan, H.; Zhou, Y.; et al. Boosting the down-shifting luminescence of rare-earth nanocrystals for biological imaging beyond 1500 nm. Nat. Commun. 2017, 8, 737. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Ma, Z.; Wang, F.; Wang, X.; Yang, Y.; Liu, Y.; Zhao, X.; Li, J.; Du, H.; Zhang, M.; et al. In vivo molecular imaging for immunotherapy using ultra-bright near-infrared-Ⅱb rare-earth nanoparticles. Nat. Biotechnol. 2019, 37, 1322–1331. [Google Scholar] [CrossRef]
- Tan, M.; Rosal, B.; Zhang, Y.; Rodríguez, E.M.; Hu, J.; Zhou, Z.; Fan, R.; Ortgies, D.H.; Fernández, N.; Chaves-Coira, I.; et al. Rare-earth-doped fluoride nanoparticles with engineered long luminescence lifetime for time-gated in vivo optical imaging in the second biological window. Nanoscale 2018, 10, 17771–17780. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wang, X.; Li, X.L.; Zeng, S.J.; Chen, G.Y. Clearable shortwave-infrared-emitting NaErF4 nanoparticles for noninvasive dynamic vascular imaging. Chem. Mater. 2020, 32, 3365–3375. [Google Scholar] [CrossRef]
- Fischer, S.; Bronstein, N.D.; Swabeck, J.K.; Chan, E.M.; Alivisatos, A.P. Precise tuning of surface quenching for luminescence enhancement in core-shell lanthanide-doped nanocrystals. Nano Lett. 2016, 16, 7241–7247. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Huang, H.; Zhang, R.; Yang, X.; Yang, H.; Li, C.; Zhang, Y.; Wang, Q.B. Activatable rare earth near-infrared-II fluorescence ratiometric nanoprobes. Nano Lett. 2021, 21, 6576–6583. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Li, X.M.; Zhou, L.; Zhang, F. Epitaxial seeded growth of rare-earth nanocrystals with efficient 800 nm near-infrared to 1525 nm short-wavelength infrared down conversion photoluminescence for in vivo bioimaging. Angew. Chem. Int. Ed. 2015, 126, 12282–12286. [Google Scholar] [CrossRef]
- Wang, Q.; Liang, T.; Wu, J.; Li, Z.; Liu, Z.H. Dye-sensitized rare earth-doped nanoparticles with boosted NIR-IIb emission for dynamic imaging of vascular network-related disorders. ACS Appl. Mater. Interfaces 2021, 13, 29303–29312. [Google Scholar] [CrossRef]
- Shao, W.; Chen, G.Y.; Kuzmin, A.; Kutscher, H.L.; Pliss, A.; Ohulchanskyy, T.Y.; Prasad, P.N. Tunable narrow band emissions from dye-sensitized core/shell/shell nanocrystals in the second near-infrared biological window. J. Am. Chem. Soc. 2016, 138, 16192–16195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Wang, D.; Kuzmin, A.; Pliss, A.; Shao, W.; Xia, J.; Qu, J.; Prasad, P.N. ICG-sensitized NaYF4:Er nanostructure for theranostics. Adv. Optical Mater. 2018, 6, 1701142. [Google Scholar] [CrossRef]
- Ren, F.; Liu, H.; Zhang, H.; Jiang, Z.; Xia, B.; Genevois, C.; He, T.; Allix, M.; Sun, Q.; Li, Z.; et al. Engineering NIR-IIb fluorescence of Er-based lanthanide nanoparticles for through-skull targeted imaging and imaging-guided surgery of orthotopic glioma. Nano Today 2020, 34, 100905. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, T.; Su, L.; Ge, X.; Chen, X.Y.; Song, J.B.; Yang, H.H. Quantum dot-based sensitization system for boosted photon absorption and enhanced second near-infrared luminescence of lanthanide-doped nanoparticle. Anal. Chem. 2020, 92, 6094–6102. [Google Scholar] [CrossRef]
- Wang, F.; Deng, R.R.; Liu, X.G. Preparation of core-shell NaGdF4 nanoparticles doped with luminescent lanthanide ions to be used as upconversion-based probes. Nat. Protoc. 2014, 9, 1634–1644. [Google Scholar] [CrossRef]
- Suter II, J.D.; Pekas, N.J.; Berry, M.T.; May, P.S. Real-time-monitoring of the synthesis of β-NaYF4:17% Yb, 3% Er nanocrystals using NIR-to-visible upconversion luminescence. J. Phys. Chem. C 2014, 118, 13238–13247. [Google Scholar] [CrossRef]
- Li, D.D.; Shao, Q.Y.; Dong, Y.; Jiang, J.Q. Phase-, shape- and size-controlled synthesis of NaYF4:Yb3+,Er3+ nanoparticles using rare-earth acetate precursors. J. Rare Earth. 2014, 32, 1032–1036. [Google Scholar] [CrossRef]
- Chen, B.; Kong, W.; Wang, N.; Zhu, G.Y.; Wang, F. Oleylamine-mediated synthesis of small NaYbF4 nanoparticles with tunable size. Chem. Mater. 2019, 31, 4779–4786. [Google Scholar] [CrossRef]
- Rastogi, C.K.; Lu, E.; Tam, J.; Pichaandi, J.M.; Howe, J.; Winnik, M.A. Influence of the sodium precursor on the cubic-to-hexagonal phase transformation and controlled preparation of uniform NaNdF4 nanoparticles. Langmuir 2021, 37, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zheng, W.; Gong, Z.; You, W.; Wei, J.; Chen, X. Rare earth ion- and transition metal ion-doped inorganic luminescent nanocrystals: From fundamentals to biodetection. Mater. Today Nano 2019, 5, 100031. [Google Scholar] [CrossRef]
- You, W.; Tu, D.; Zheng, W.; Shang, X.; Song, X.; Zhou, S.; Liu, Y.; Li, R.; Chen, X. Large-scale synthesis of uniform lanthanide-doped NaREF4 upconversion/ downshifting nanoprobes for bioapplications. Nanoscale 2018, 10, 11477–11484. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Ge, H.; Wei, Y.; Zhang, K.; Su, W.; Zhou, J.; Yin, L.; Zhan, Q.; Jing, S.; Huang, L. Design for brighter photon upconversion emissions via energy level overlap of lanthanide ions. ACS Nano 2018, 12, 10992–10999. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gai, S.; Niu, N.; He, F.; Yang, P.P. Synthesis of NaYF4 microcrystals with different morphologies and enhanced up-conversion luminescence properties. Phys. Chem. Chem. Phys. 2013, 15, 16795. [Google Scholar] [CrossRef]
- Wang, L.; Li, Y.D. Controlled synthesis and luminescence of lanthanide doped NaYF4 nanocrystals. Chem. Mater. 2007, 19, 727–734. [Google Scholar] [CrossRef]
- Sun, L.; Wei, R.; Feng, J.; Zhang, H.J. Tailored lanthanide-doped upconversion nanoparticles and their promising bioapplication prospects. Coordin. Chem. Rev. 2018, 364, 10–32. [Google Scholar] [CrossRef]
- Qu, X.; Pan, G.; Yang, H.K.; Chen, Y.; Chung, J.W.; Moon, B.K.; Choi, B.C.; Jeong, J.H. Solvothermal synthesis and luminescence properties of NaYF4:Ln3+ (Eu3+, Tb3+, Yb3+/Er3+) nano- and microstructures. Opt. Mater. 2012, 34, 1007–1012. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, W.; Kong, J.; Liu, Y.; Huang, P.; Zhou, S.; Chen, Z.; Shi, J.; Chen, X. Rechargeable and LED-activated ZnGa2O4: Cr3+ near-infrared persistent luminescence nanoprobes for background-free biodetection. Nanoscale 2017, 9, 6846–6853. [Google Scholar] [CrossRef]
- Cao, T.M.D.; Le, T.T.G.; Nguyen, T.P.N.; Dau, T.A.N.; Nguyen, V.T.; Tran, T.T.V. Investigating the effect of Yb3+ and Er3+ concentration on red/green luminescent ratio in β-NaYF4: Er, Yb nanocrystals using spectroscopic techniques. J. Mol. Struct. 2020, 12, 128014. [Google Scholar] [CrossRef]
- Carniato, F.; Thangavel, K.; Tei, L.; Botta, M. Structure and dynamics of the hydration shells of citrate-coated GdF3 nanoparticles. J. Mater. Chem. B 2013, 1, 2442. [Google Scholar] [CrossRef] [PubMed]
- Evanics, F.; Diamente, P.R.; van Veggel, F.C.J.M.; Stanisz, G.J.; Prosser, R.S. Water-soluble GdF3 and GdF3/LaF3 nanoparticless-physical characterization and NMR relaxation properties. Chem. Mater. 2006, 18, 2499. [Google Scholar] [CrossRef]
- Bednarkiewicz, A.; Mech, A.; Karbowiak, M.; Strek, W. Spectral properties of Eu3+ doped NaGdF4 nanocrystals. J. Lumin. 2005, 114, 247. [Google Scholar] [CrossRef]
- Loo, J.F.C.; Chien, Y.H.; Yin, F.; Kong, S.K.; Ho, H.P.; Yong, K.T. Upconversion and downconversion nanoparticles for biophotonics and nanomedicine. Coord. Chem. Rev. 2019, 400, 213042. [Google Scholar] [CrossRef]
- Donato, G.; Grosvenor, A.P. Crystallization of rare-earth phosphate-borosilicate glass composites synthesized by a one-step coprecipitation method. Cryst. Growth Des. 2020, 20, 2217–2231. [Google Scholar] [CrossRef]
- Aleshin, D.K.; Mashkovtsev, M.A.; Kuznetsova, Y.A.; Rychkov, V.N.; Zatsepin, A.F.; Gordeev, E.V. Fabrication of (Y0.95Eu0.05)2O3 phosphors with enhanced properties by coprecipitation of layered rare earth hydroxide. J. Alloys Compd. 2019, 805, 258–266. [Google Scholar] [CrossRef]
- Yi, G.S.; Chow, G.M. Colloidal LaF3: Yb, Er, LaF3: Yb, Ho and LaF3: Yb, Tm nanocrystals with multicolor upconversion fluorescence. J. Mater. Chem. 2005, 15, 4460–4464. [Google Scholar] [CrossRef]
- Guan, H.; Feng, Y.; Zhang, W.; Wang, W.; Hu, Y. Room-temperature facile synthesis of hexagonal NaYF4 and NaYF4: Yb, Er powder without any organic additives and its upconversion fluorescence properties. Adv. Powder Technol. 2022, 33, 103381. [Google Scholar] [CrossRef]
- Sarkar, D.; Meesaragandla, B.; Samanta, T.; Mahalingam, V. A greener approach towards making highly luminescent Ln3+-doped NaYF4 nanoparticles with ligand-assisted phase control. ChemistrySelect 2016, 1, 4785–4793. [Google Scholar] [CrossRef]
- Wang, M.; Mi, C.C.; Wang, S.; Li, F.; Liu, J.L.; Xu, S.K. Synthesis and characterization of NaYF4: Yb, Er upconversion fluorescent nanoparticles via a co-precipitation method. Spectrosc. Spect. Anal. 2009, 29, 3327–3331. [Google Scholar]
- Sekiyama, S.; Umezawa, M.; Kuraoka1, S.; Ube, T.; Kamimura, M.; Soga, K. Temperature sensing of deep abdominal region in mice by using over-1000 nm near-infrared luminescence of rare-earth-doped NaYF4 nanothermometer. Sci. Rep. 2018, 8, 16979. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Gu, Y.Y.; Chai, Y.; Ke, J.; Liu, Y.; Xu, X.; Li, Z.X.; Feng, W.; Li, F.Y. Luminescence interference-free lifetime nanothermometry pinpoints in vivo temperature. Sci. China Chem. 2021, 64, 974–984. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, H.; Jia, M.; Jin, X.; Lv, Z.; Dai, M.; Zhu, K.M.; Feng, J.; Ge, X.; Fu, Z. Multifunctional lanthanide ions-doped Ba2TiGe2O8 phosphor for near-infrared ratiometric thermometer and information security. J. Lumin. 2022, 243, 118652. [Google Scholar] [CrossRef]
- Nexha, A.; Carvajal, J.J.; Pujol, M.C.; Díaza, F.; Aguiló, M. Lanthanide doped luminescence nanothermometers in the biological windows: Strategies and applications. Nanoscale 2021, 13, 7913–7987. [Google Scholar] [CrossRef]
- Lei, X.; Li, R.; Tu, D.; Shang, X.Y.; Liu, Y.; You, W.; Sun, C.; Zhang, F.; Chen, X.Y. Intense near-infrared-Ⅱ luminescence from NaCeF4: Er/Yb nanoprobes for in vitro bioas-say and in vivo bioimaging. Chem. Sci. 2018, 9, 4682–4688. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Niu, M.; Wang, W.; Su, L.; Feng, H.J.; Lin, H.X.; Ge, X.; Wu, R.; Li, Q.; Liu, J.; et al. In situ activatable ratiometric NIR-II fluorescence nanoprobe for quantitative detection of H2S in colon cancer. Anal. Chem. 2021, 93, 9356–9363. [Google Scholar] [CrossRef]
- Jia, Q.; Liu, Y.; Duan, Y.; Zhou, J. Interference-free detection of hydroxyl radical and arthritis diagnosis by rare earth-based nanoprobe utilizing SWIR emission as reference. Anal. Chem. 2019, 91, 11433–11439. [Google Scholar] [CrossRef]
- Liao, N.; Su, L.; Zheng, Y.; Zhao, B.; Wu, M.; Zhang, D.; Yang, H.H.; Liu, X.L.; Song, J.B. In vivo tracking of cell viability for adoptive natural killer cell-based immunotherapy by ratiometric NIR-II fluorescence imaging. Angew. Chem. Int. Ed. 2021, 133, 2–11. [Google Scholar] [CrossRef]
- Liao, N.; Su, L.; Cao, Y.; Qiu, L.; Xie, R.; Peng, F.; Cai, Z.; Liu, X.; Song, J.; Zeng, Y. Tracking cell viability for adipose-derived mesenchymal stem cell-based therapy by quantitative fluorescence imaging in the second near-infrared window. ACS Nano 2022, 16, 2889–2900. [Google Scholar] [CrossRef]
- Wang, S.; Liu, L.; Fan, Y.; El-Toni, A.M.; Alhoshan, M.S.; Li, D.; Zhang, F. In vivo high-resolution ratiometric fluorescence imaging of inflammation using NIR-II nanoprobes with 1550 nm emission. Nano Lett. 2019, 19, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Z.; Wang, C.; Wu, Y.T.; Li, Z.; Liu, Z.H. Visualizing oxidative stress level for timely assessment of ischemic stroke via a ratiometric near-infrared-II luminescent nanoprobe. ACS Nano 2021, 15, 11940–11952. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Zhou, X.B.; Xue, M.; Han, C.; Feng, W.; Li, F.Y. Dual near-infrared-emissive luminescent nanoprobes for ratiometric luminescent monitoring of ClO− in living organisms. ACS Appl. Mater. Interfaces 2019, 11, 15298–15305. [Google Scholar] [CrossRef]
- Ge, X.; Lou, Y.; Su, L.; Chen, B.; Guo, Z.Y.; Gao, S.; Zhang, W.; Chen, T.; Song, J.B.; Yang, H.H. Single wavelength laser excitation ratiometric NIR-II fluorescent probe for molecule imaging in vivo. Anal. Chem. 2020, 92, 6111–6120. [Google Scholar] [CrossRef] [PubMed]
- Pei, P.; Hu, H.; Chen, Y.; Wang, S.; Chen, J.; Ming, J.; Yang, Y.; Sun, C.; Zhao, S.; Zhang, F. NIR-II ratiometric lanthanide-dye hybrid nanoprobes doped bioscaffolds for in situ bone repair monitoring. Nano Lett. 2022, 22, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, H.; Ge, X.; Mu, J.; Su, L.; Zhang, X.; Niu, M.; Yang, H.H.; Song, J.B. Dye-sensitized downconversion nanoprobes with emission beyond 1500 nm for ratiometric visualization of cancer redox state. Adv. Funct. Mater. 2021, 31, 2009942. [Google Scholar] [CrossRef]
- Li, Z.; Wu, J.; Wang, Q.; Liang, T.; Ge, J.; Wang, P.; Liu, Z. A universal strategy to construct lanthanide-doped nanoparticles-based activable NIR-II luminescence probe for bioimaging. iScience 2020, 23, 100962. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Li, B.H.; Wu, Y.; He, H.; Zhu, X.; Zhang, H.; Dou, C.; Feng, L.; Fan, Y.; Zhang, F. A tumor-microenvironment-responsive lanthanide-cyanine FRET sensor for NIR-II luminescence-lifetime in situ imaging of hepatocellular carcinoma. Adv. Mater. 2020, 32, 2001172. [Google Scholar] [CrossRef]
- Zhong, Y.; Gu, J.; Su, Y.; Zhao, L.; Zhou, Y.; Peng, J. Real-time screening of hepatotoxins in natural medicine by peroxynitrite responsive lanthanide-based NIR-II luminescent probes. Chem. Eng. J. 2022, 433, 133263. [Google Scholar] [CrossRef]
- Liu, Q.; Zhong, Y.; Su, Y.; Zhao, L.Z.; Peng, J.J. Real-time imaging of hepatic inflammation using hydrogen sulfide-activatable second near-infrared luminescent nanoprobes. Nano Lett. 2021, 21, 4606–4614. [Google Scholar] [CrossRef]
- Deng, Z.; Bi, S.; Jiang, M.Y.; Zeng, S.J. Endogenous H2S-activated orthogonal second near-infrared emissive nanoprobe for in situ ratiometric fluorescence imaging of metformin-induced liver injury. ACS Nano 2021, 15, 3201–3211. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhou, L.; Wang, W.; Li, X.M.; Zhang, F. In vivo gastrointestinal drug-release monitoring through second near-infrared window fluorescent bioimaging with orally delivered microcarriers. Nat. Commun. 2017, 8, 14702. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Ye, L.; Gu, J.; Zhao, L.; Zhou, Y.; Peng, J. Sensing and imaging of PPi in vivo using lanthanide-based second near-infrared luminescent probes. J. Mater. Chem. B 2022, 10, 1055. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Li, W.F.; Liu, H.H.; Zhang, Y.J.; Chen, G.C.; Li, Z.J.; Wang, Q.B. An activatable NIR-II nanoprobe for in vivo early real-time diagnosis of traumatic brain injury. Angew. Chem. Int. Ed. 2020, 59, 247–252. [Google Scholar] [CrossRef]
- Wu, Z.F.; Ke, J.X.; Liu, Y.S.; Sun, P.M.; Hong, M.C. Lanthanide-based NIR-II fluorescent nanoprobes and their biomedical applications. Acta Chim. Sin. 2022, 80, 542–552. [Google Scholar] [CrossRef]










| NIR-II LnNCs | Excitation (nm) | Emission (nm) | Response Unit | Influence Signal | Applications | Ref. |
|---|---|---|---|---|---|---|
| NaCeF4: Er, Yb | 980 | 1530 | Ce3+ → Ce4+ | 1530 | H2O2 | [86] |
| NaErF4: 2%Ho@NaYF4 | 1530 | 980/1180 | IR1061 | 980 | H2O2 | [44] |
| NaErF4@NaLuF4 | 980 | 654/1550 | Cypate | 654 | ·OH | [88] |
| NaYF4: 18%Yb3+, 2%Er3+ | 808/980 | 1550 | IR786s | 808/1550 | ROS | [89] |
| NaYF4: 20% Yb and 2%Er@NaYF4 | 808/980 | 1550 | IR786s | 808/1550 | ROS | [90] |
| NaYF4: 50%Er@NaYF4 | 808/980 | 1550 | Cy7.5 | 808/1550 | HOCl | [91] |
| NaYbF4: 5%Er, 5%Ce@NaYF4: 20%Nd | 808/980 | 1550 | IR-783 | 808/1550 | HOCl and ·OH | [92] |
| NaYbF4: Er@NaYF4: Yb@NaYF4: Nd | 808 | 925/1525 | Cy925 | 925 | HOCl | [93] |
| NaYF4: 18%Yb3+, 2%Er3+ | 980 | 1150/1550 | SeTT | 1150 | HOCl | [94] |
| NaErF4@NaYF4 | 808/980 | 1525 | IR808 | 808/1525 | HOCl | [95] |
| NaYF4: 20%Yb, 2%Er@NaYF4: 30%Nd | 808/980 | 1550 | 4-nitrophenol-Cy7 | 808/1550 | GSH | [96] |
| NaYF4: 20%Yb, 2%Er@NaYF4: 30%Nd | 808 | 1530 | HC-Ni | 1530 | GSH | [97] |
| NaYF4@NaYF4: 1%Nd3+ | 808 | 1064 | MY-1057 | 1060 | ONOO− | [98] |
| NaErF4@NaYF4@NaYF4: 10%Nd@NaYF4 | 808 | 1060/1525 | A1094 | 1060 | ONOO− | [51] |
| NaGdF4: 3%Nd@NaGdF4 | 808 | 1060 | Compound 1 | 1060 | ONOO− | [99] |
| NaYF4: 20%Yb, 2%Er@NaYF4 | 808/980 | 1050/1550 | Ag+ → Ag2S | 808/1050 | H2S | [87] |
| NaGdF4: 2%Nd@NaGdF4 | 808 | 1060 | Compound 1 | 1060 | H2S | [100] |
| NaYF4: Gd, Yb, Er@NaYF4: Yb | 808/980 | 1053/1525 | Ag → Ag2S | 808/1053 | H2S | [101] |
| NaGdF4: 5%Nd@NaGdF4 | 730/808 | 1064 | BSA-NPTAT | 730/1064 | BSA drug-release monitoring | [102] |
| NaGdF4:Nd@NaGdF4 | 808 | 1058 | TA-Fe3+ | 1058 | PPi | [103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, T.; Qin, J.; Zhang, J.; Guo, L.; Yang, M.; Wu, X.; You, M.; Peng, H. Recent Progresses in NIR-II Luminescent Bio/Chemo Sensors Based on Lanthanide Nanocrystals. Chemosensors 2022, 10, 206. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors10060206
Yang T, Qin J, Zhang J, Guo L, Yang M, Wu X, You M, Peng H. Recent Progresses in NIR-II Luminescent Bio/Chemo Sensors Based on Lanthanide Nanocrystals. Chemosensors. 2022; 10(6):206. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors10060206
Chicago/Turabian StyleYang, Tingyu, Jinglei Qin, Jinling Zhang, Lanying Guo, Mu Yang, Xi Wu, Mei You, and Hongshang Peng. 2022. "Recent Progresses in NIR-II Luminescent Bio/Chemo Sensors Based on Lanthanide Nanocrystals" Chemosensors 10, no. 6: 206. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors10060206