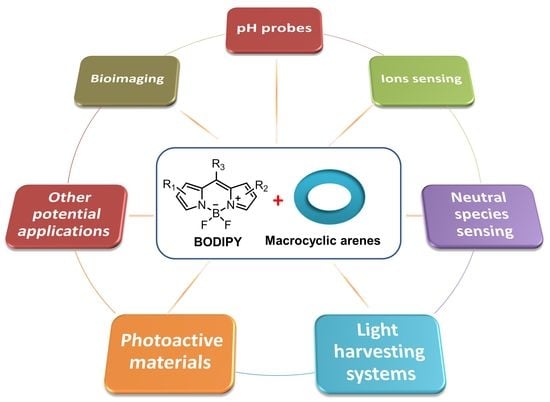
Macrocyclic Arenes Functionalized with BODIPY: Rising Stars among Chemosensors and Smart Materials
Abstract
:1. Introduction
2. Calixarenes with BODIPY Functionalities
2.1. s-Block Metal Ion Sensing
2.2. Transition Metal Ion Sensing
2.3. Fluorescence Sensing of pH
2.4. Other Applications
3. Resorcinarenes Functionalized with BODIPY
4. Pillararenes Substituted with BODIPY
4.1. Chemosensors
4.2. Supramolecular Assembly Systems
4.3. Mimicking Light Harvesting System
5. Summary and Outlook
Funding
Acknowledgments
Conflicts of Interest
List of Abbreviations
| BODIPY | Boron-dipyrromethene, 4,4-difluoro-4-bora-3a, 4a-diaza-s-indacenes |
| CT | Charge transfer |
| CuAAC | Copper-Catalyzed azide/alkyne cycloaddition |
| 2DES | Two-dimensional electronic spectroscopy |
| DDQ | 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone |
| DFT | Density functional theory |
| DMF | N,N-dimethylformamide |
| DMSO | Dimethylsulfoxide |
| DOX | Doxorubicin |
| EET | Electronic energy transfer |
| ESIPT | Excited-state intermolecular proton transfer |
| FRET | Fluorescence resonance energy transfer |
| 1H NMR | Proton nuclear magnetic resonance |
| ICT | Intramolecular charge transfer |
| IUPAC | International Union of Pure and Applied Chemistry |
| MD | Molecular dynamics |
| MLCT | Metal-to-ligand charge transfer |
| NIR | Near infrared |
| PDT | Photodynamic therapy |
| PEG | Polyethylene glycol |
| PET | Photo-induced electron transfer |
| PVC | Polyvinyl chloride |
| ROS | Reactive oxygen species |
| SEM | Scanning electron microscope |
| TDMACl | Tridodecylmethylammonium chloride |
| TD-DFT | Time dependent-density functional theory |
| TFA | Trifluoroacetic acid |
| VT FRET | Variable-temperature fluorescence resonance energy transfer |
| VT NMR | Variable-temperature nuclear magnetic resonance |
References
- Atwood, J.L. Comprehensive Supramolecular Chemistry II.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Diederich, F.; Stang, P.J.; Tykwinski, R.R. Modern Supramolecular Chemistry: Strategies for Macrocycle Synthesis; John Wiley & Sons: New York, NY, USA, 2008. [Google Scholar]
- Li, J.; Yim, D.; Jang, W.-D.; Yoon, J. Recent progress in the design and applications of fluorescence probes containing crown ethers. Chem. Soc. Rev. 2017, 46, 2437–2458. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sharma, A.; Singh, H.; Suating, P.; Kim, H.S.; Sunwoo, K.; Shim, I.; Gibb, B.C.; Kim, J.S. Revisiting Fluorescent Calixarenes: From Molecular Sensors to Smart Materials. Chem. Rev. 2019, 119, 9657–9721. [Google Scholar] [CrossRef]
- Biros, S.M.; Rebek, J., Jr. Structure and binding properties of water-soluble cavitands and capsules. Chem. Soc. Rev. 2007, 36, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Lü, J.; Lin, J.-X.; Cao, M.-N.; Cao, R. Cucurbituril: A promising organic building block for the design of coordination compounds and beyond. Coordin. Chem. Rev. 2013, 257, 1334–1356. [Google Scholar] [CrossRef]
- Chen, C.F.; Han, Y. Triptycene-Derived Macrocyclic Arenes: From Calixarenes to Helicarenes. Acc. Chem. Res. 2018, 51, 2093–2106. [Google Scholar] [CrossRef] [PubMed]
- Lindoy, L.F.; Park, K.M.; Lee, S.S. Metals, macrocycles and molecular assemblies-macrocyclic complexes in metallo-supramolecular chemistry. Chem. Soc. Rev. 2013, 42, 1713–1727. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Shao, Z. Self-Assembling Organic Nanotubes with Precisely Defined, Sub-nanometer Pores: Formation and Mass Transport Characteristics. Acc. Chem. Res. 2013, 46, 2856–2866. [Google Scholar] [CrossRef]
- Han, X.N.; Han, Y.; Chen, C.F. Pagoda[4]arene and i-Pagoda[4]arene. J. Am. Chem. Soc. 2020, 142, 8262–8269. [Google Scholar] [CrossRef]
- Della Sala, P.; Del Regno, R.; Talotta, C.; Capobianco, A.; Hickey, N.; Geremia, S.; De Rosa, M.; Spinella, A.; Soriente, A.; Neri, P.; et al. Prismarenes: A New Class of Macrocyclic Hosts Obtained by Templation in a Thermodynamically Controlled Synthesis. J. Am. Chem. Soc. 2020, 142, 1752–1756. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Zhang, Z.Y.; Yu, C.; Wang, B.; Dong, M.; Zeng, X.; Gou, R.; Cui, L.; Li, C. A Modular Synthetic Strategy for Functional Macrocycles. Angew. Chem. Int. Ed. 2020, 59, 7214–7218. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Samanta, K.; Wan, X.; Thikekar, T.U.; Chao, Y.; Li, S.; Du, K.; Xu, J.; Gao, Y.; Zuilhof, H.; et al. Tiara[5]arenes: Synthesis, Solid-State Conformational Studies, Host-Guest Properties, and Application as Nonporous Adaptive Crystals. Angew. Chem. Int. Ed. 2020, 59, 3994–3999. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.P.; Jiang, W. Prismarene: An Emerging Naphthol-Based Macrocyclic Arene. Angew. Chem. Int. Ed. 2020, 59. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, K.; Li, B.; Cui, L.; Li, J.; Jia, X.; Zhao, H.; Fang, J.; Li, C. Efficient Separation of cis- and trans-1,2-Dichloroethene Isomers by Adaptive Biphen[3]arene Crystals. Angew. Chem. Int. Ed. 2019, 58, 10281–10284. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, B.; Huang, X.; Dai, L.; Cui, L.; Li, J.; Jia, X.; Li, C. Terphen[n]arenes and Quaterphen[n]arenes (n = 3–6): One-Pot Synthesis, Self-Assembly into Supramolecular Gels, and Iodine Capture. Angew. Chem. Int. Ed. 2019, 58, 3885–3889. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.-C.; Hu, X.-Y.; Guo, D.-S. Biomedical Applications of Calixarenes: State-of-the-Art and Perspectives. Angew. Chem. Int. Ed. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, M.H.; Mutihac, L.; Vicens, J.; Kim, J.S. Host-guest sensing by calixarenes on the surfaces. Chem. Soc. Rev. 2012, 41, 1173–1190. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Kim, T. Biological applications of functionalized calixarenes. Chem. Soc. Rev. 2013, 42, 366–386. [Google Scholar] [CrossRef]
- Tian, H.-W.; Liu, Y.-C.; Guo, D.-S. Assembling features of calixarene-based amphiphiles and supra-amphiphiles. Mater. Chem. Front. 2020, 4, 46–98. [Google Scholar] [CrossRef]
- Timmerman, P.; Verboom, W.; Reinhoudt, D.N. Resorcinarenes. Tetrahedron 1996, 52, 2663–2704. [Google Scholar] [CrossRef] [Green Version]
- Gramage-Doria, R.; Armspach, D.; Matt, D. Metallated cavitands (calixarenes, resorcinarenes, cyclodextrins) with internal coordination sites. Coordin. Chem. Rev. 2013, 257, 776–816. [Google Scholar] [CrossRef]
- Pochorovski, I.; Diederich, F. Fluorophore-Functionalized and Top-Covered Resorcin[4]arene Cavitands. Isr. J. Chem. 2012, 52, 20–29. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, J.-M.; Rebek, J. Molecules in Confined Spaces: Reactivities and Possibilities in Cavitands. Chem 2020. [Google Scholar] [CrossRef]
- Pinalli, R.; Pedrini, A.; Dalcanale, E. Environmental Gas Sensing with Cavitands. Chem. Eur. J. 2018, 24, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Yamanaka, M. Self-assembled capsules based on tetrafunctionalized calix[4]resorcinarene cavitands. Chem. Soc. Rev. 2015, 44, 449–466. [Google Scholar] [CrossRef] [PubMed]
- Pochorovski, I.; Diederich, F. Development of redox-switchable resorcin[4]arene cavitands. Acc. Chem. Res. 2014, 47, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, N.; Brenner, E.; Sémeril, D.; Matt, D.; Harrowfield, J. The Use of Resorcinarene Cavitands in Metal-Based Catalysis. Eur. J. Org. Chem. 2017, 6100–6113. [Google Scholar] [CrossRef]
- Murray, J.; Kim, K.; Ogoshi, T.; Yao, W.; Gibb, B.C. The aqueous supramolecular chemistry of cucurbit[n]urils, pillar[n]arenes and deep-cavity cavitands. Chem. Soc. Rev. 2017, 46, 2479–2496. [Google Scholar] [CrossRef] [Green Version]
- Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.-a.; Nakamoto, Y. para-Bridged Symmetrical Pillar[5]arenes: Their Lewis Acid Catalyzed Synthesis and Host–Guest Property. J. Am. Chem. Soc. 2008, 130, 5022–5023. [Google Scholar] [CrossRef]
- Xue, M.; Yang, Y.; Chi, X.; Zhang, Z.; Huang, F. Pillararenes, a new class of macrocycles for supramolecular chemistry. Acc. Chem. Res. 2012, 45, 1294–1308. [Google Scholar] [CrossRef]
- Ogoshi, T.; Yamagishi, T.A.; Nakamoto, Y. Pillar-Shaped Macrocyclic Hosts Pillar[n]arenes: New Key Players for Supramolecular Chemistry. Chem. Rev. 2016, 116, 7937–8002. [Google Scholar] [CrossRef] [PubMed]
- Si, W.; Xin, P.; Li, Z.T.; Hou, J.L. Tubular Unimolecular Transmembrane Channels: Construction Strategy and Transport Activities. Acc. Chem. Res. 2015, 48, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Jie, K.; Zhou, Y.; Li, E.; Huang, F. Nonporous Adaptive Crystals of Pillararenes. Acc. Chem. Res. 2018, 51, 2064–2072. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Z.; Zhao, Y. Pillararene-based self-assembled amphiphiles. Chem. Soc. Rev. 2018, 47, 5491–5528. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Jin, M.; Yang, K.; Pei, Y.; Pei, Z. Supramolecular delivery systems based on pillararenes. Chem. Commun. 2018, 54, 13626–13640. [Google Scholar] [CrossRef]
- Song, N.; Lou, X.Y.; Ma, L.; Gao, H.; Yang, Y.W. Supramolecular nanotheranostics based on pillarenes. Theranostics 2019, 9, 3075–3093. [Google Scholar] [CrossRef] [PubMed]
- Fa, S.; Kakuta, T.; Yamagishi, T.-a.; Ogoshi, T. One-, Two-, and Three-Dimensional Supramolecular Assemblies Based on Tubular and Regular Polygonal Structures of Pillar[n]arenes. CCS Chem. 2019, 1, 50–63. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Yang, Y.; Xu, F.; Liang, T.; Wen, H.; Tian, W. Pillararene-based supramolecular polymers. Chem. Commun. 2019, 55, 271–285. [Google Scholar] [CrossRef]
- Chen, L.; Cai, Y.; Feng, W.; Yuan, L. Pillararenes as macrocyclic hosts: A rising star in metal ion separation. Chem. Commun. 2019, 55, 7883–7898. [Google Scholar] [CrossRef]
- Fang, Y.; Deng, Y.; Dehaen, W. Tailoring pillararene-based receptors for specific metal ion binding: From recognition to supramolecular assembly. Coordin. Chem. Rev. 2020, 415, 213313. [Google Scholar] [CrossRef]
- Banuelos, J. BODIPY Dye, the Most Versatile Fluorophore Ever? Chem. Rec. 2016, 16, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Treibs, A.; Kreuzer, F.-H. Difluorboryl-Komplexe von Di- und Tripyrrylmethenen. Justus Liebigs Ann. Chem. 1968, 718, 208–223. [Google Scholar] [CrossRef]
- Shah, M.; Thangaraj, K.; Soong, M.L.; Wolford, L.T.; Boyer, J.H.; Politzer, I.R.; Pavlopoulos, T.G. Pyrromethene–BF2 complexes as laser dyes: 1. Heteroat. Chem. 1990, 1, 389–399. [Google Scholar] [CrossRef]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent indicators based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef] [PubMed]
- Boens, N.; Verbelen, B.; Dehaen, W. Postfunctionalization of the BODIPY Core: Synthesis and Spectroscopy. Eur. J. Org. Chem. 2015, 6577–6595. [Google Scholar] [CrossRef] [Green Version]
- Boodts, S.; Fron, E.; Hofkens, J.; Dehaen, W. The BOPHY fluorophore with double boron chelation: Synthesis and spectroscopy. Coordin. Chem. Rev. 2018, 371, 1–10. [Google Scholar] [CrossRef]
- Boens, N.; Verbelen, B.; Ortiz, M.J.; Jiao, L.; Dehaen, W. Synthesis of BODIPY dyes through postfunctionalization of the boron dipyrromethene core. Coordin. Chem. Rev. 2019, 399, 213024. [Google Scholar] [CrossRef]
- Ni, Y.; Wu, J. Far-red and near infrared BODIPY dyes: Synthesis and applications for fluorescent pH probes and bio-imaging. Org. Biomol. Chem. 2014, 12, 3774–3791. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, N.; Ji, X.; Tao, Y.; Wang, J.; Zhao, W. BODIPY-Based Fluorescent Probes for Biothiols. Chem. Eur. J. 2020, 26, 4172–4192. [Google Scholar] [CrossRef]
- Kolemen, S.; Akkaya, E.U. Reaction-based BODIPY probes for selective bio-imaging. Coordin. Chem. Rev. 2018, 354, 121–134. [Google Scholar] [CrossRef]
- Kowada, T.; Maeda, H.; Kikuchi, K. BODIPY-based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2015, 44, 4953–4972. [Google Scholar] [CrossRef] [PubMed]
- Nepomnyashchii, A.B.; Bard, A.J. Electrochemistry and electrogenerated chemiluminescence of BODIPY dyes. Acc. Chem. Res. 2012, 45, 1844–1853. [Google Scholar] [CrossRef] [PubMed]
- Bessette, A.; Hanan, G.S. Design, synthesis and photophysical studies of dipyrromethene-based materials: Insights into their applications in organic photovoltaic devices. Chem. Soc. Rev. 2014, 43, 3342–3405. [Google Scholar] [CrossRef] [PubMed]
- Kamkaew, A.; Lim, S.H.; Lee, H.B.; Kiew, L.V.; Chung, L.Y.; Burgess, K. BODIPY dyes in photodynamic therapy. Chem. Soc. Rev. 2013, 42, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, B.; Passador, K.; Goze, C.; Denat, F.; Bodio, E.; Salmain, M. Metal-based BODIPY derivatives as multimodal tools for life sciences. Coordin. Chem. Rev. 2018, 358, 108–124. [Google Scholar] [CrossRef] [Green Version]
- Turksoy, A.; Yildiz, D.; Akkaya, E.U. Photosensitization and controlled photosensitization with BODIPY dyes. Coordin. Chem. Rev. 2019, 379, 47–64. [Google Scholar] [CrossRef]
- Kim, T.; Duan, Z.; Talukdar, S.; Lei, C.; Kim, D.; Sessler, J.L.; Sarma, T. Excitonically Coupled Cyclic BF2 Arrays of Calix[8]- and Calix[16]phyrin as Near-IR-Chromophores. Angew. Chem. Int. Ed. 2020. [Google Scholar] [CrossRef]
- Ke, X.S.; Kim, T.; Lynch, V.M.; Kim, D.; Sessler, J.L. Flattened Calixarene-like Cyclic BODIPY Array: A New Photosynthetic Antenna Model. J. Am. Chem. Soc. 2017, 139, 13950–13956. [Google Scholar] [CrossRef]
- Ishida, M.; Omagari, T.; Hirosawa, R.; Jono, K.; Sung, Y.M.; Yasutake, Y.; Uno, H.; Toganoh, M.; Nakanotani, H.; Fukatsu, S. Boron Difluoride Complexes of Expanded N-Confused Calix[n]phyrins That Demonstrate Unique Luminescent and Lasing Properties. Angew. Chem. Int. Ed. 2016, 55, 12045–12049. [Google Scholar] [CrossRef]
- Zhang, F.; Baudron, S.A.; Hosseini, M.W. Solvent and anion effects on the organization of a luminescent [2+2] BODIPY/Ag(I) metallamacrocycle in the crystalline state. CrystEngComm 2017, 19, 4393–4400. [Google Scholar] [CrossRef]
- Chua, M.H.; Kim, T.; Lim, Z.L.; Gopalakrishna, T.Y.; Ni, Y.; Xu, J.; Kim, D.; Wu, J. BODIPY-Based Antiaromatic Macrocycles: Facile Synthesis by Knoevenagel Condensation and Unusual Aggregation-Enhanced Two-Photon Absorption Properties. Chem. Eur. J. 2018, 24, 2232–2241. [Google Scholar] [CrossRef] [PubMed]
- Hojo, T.; Nakamura, T.; Matsuoka, R.; Nabeshima, T. Uniquely folded shapes, photophysical properties, and recognition abilities of macrocyclic BODIPY oligomers. Heteroatom Chem. 2018, 29, e21470. [Google Scholar] [CrossRef] [Green Version]
- Uchida, J.; Nakamura, T.; Yamamura, M.; Yamaguchi, G.; Nabeshima, T. m-Phenylene-Linked Dipyrrins and Their Boron-Difluoride Complexes as Variously Shaped Macrocyclic Oligomers. Org. Lett. 2016, 18, 5380–5383. [Google Scholar] [CrossRef] [PubMed]
- Cha, N.R.; Moon, S.Y.; Chang, S.-K. New ON–OFF type Ca2+-selective fluoroionophore having boron–dipyrromethene fluorophores. Tetrahedron Lett. 2003, 44, 8265–8268. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.S. BODIPY appended cone-calix[4]arene: Selective fluorescence changes upon Ca2+ binding. Tetrahedron Lett. 2006, 47, 7051–7055. [Google Scholar] [CrossRef]
- Malval, J.-P.; Leray, I.; Valeur, B. A highly selective fluorescent molecular sensor for potassium based on a calix[4]bisazacrown bearing boron-dipyrromethene fluorophores. New J. Chem. 2005, 29, 1089–1094. [Google Scholar] [CrossRef]
- Melnikov, P.; Zanoni, L.Z. Clinical Effects of Cesium Intake. Biol. Trace Elem. Res. 2010, 135, 1–9. [Google Scholar] [CrossRef]
- Depauw, A.; Kumar, N.; Ha-Thi, M.-H.; Leray, I. Calixarene-Based Fluorescent Sensors for Cesium Cations Containing BODIPY Fluorophore. J. Phys. Chem. A 2015, 11, 6065–6073. [Google Scholar] [CrossRef]
- Csokai, V.; Kádár, M.; Ha Mai, D.L.; Varga, O.; Tóth, K.; Kubinyi, M.; Grün, A.; Bitter, I. Synthesis, optical and electroanalytical characterizations of a thiacalix[4](N-phenylazacrown-5)ether–BODIPY ionophore. Tetrahedron 2008, 64, 1058–1063. [Google Scholar] [CrossRef]
- Dam, H.H.; Reinhoudt, D.N.; Verboom, W. Multicoordinate ligands for actinide/lanthanide separations. Chem. Soc. Rev. 2007, 36, 367–377. [Google Scholar] [CrossRef]
- Bayrakcı, M.; Kursunlu, A.N.; Güler, E.; Ertul, Ş. A new calix[4]azacrown ether based boradiazaindacene (BODIPY): Selective fluorescence changes towards trivalent lanthanide ions. Dyes Pigments 2013, 99, 268–274. [Google Scholar] [CrossRef]
- Nolan, E.M.; Lippard, S.J. Tools and tactics for the optical detection of mercuric ion. Chem. Rev. 2008, 108, 3443–3480. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, N. Noble Metal Nanoparticles-Based Colorimetric Biosensor for Visual Quantification: A Mini Review. Chemosensors 2019, 7, 53. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.D.; Curtis, R.M.; Wallace, K.J. Low Molecular Weight Fluorescent Probes (LMFPs) to Detect the Group 12 Metal Triad. Chemosensors 2019, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Sulak, M.; Kursunlu, A.N.; Girgin, B.; Karakuş, Ö.Ö.; Güler, E. A highly selective fluorescent sensor for mercury(II) ion based on BODIPY and Calix[4]arene bearing triazolenaphthylene groups; synthesis and photophysical investigations. J. Photochem. Photobio. A 2017, 349, 129–137. [Google Scholar] [CrossRef]
- Hou, J.-T.; Ren, W.X.; Li, K.; Seo, J.; Sharma, A.; Yu, X.-Q.; Kim, J.S. Fluorescent bioimaging of pH: From design to applications. Chem. Soc. Rev. 2017, 46, 2076–2090. [Google Scholar] [CrossRef]
- Yue, Y.; Huo, F.; Lee, S.; Yin, C.; Yoon, J. A review: The trend of progress about pH probes in cell application in recent years. Analyst 2017, 142, 30–41. [Google Scholar] [CrossRef]
- Méndez-Ardoy, A.; Reina, J.J.; Montenegro, J. Synthesis and Supramolecular Functional Assemblies of Ratiometric pH Probes. Chem. Eur. J. 2020, 26, 7516–7536. [Google Scholar] [CrossRef]
- Hammarling, K.; Engholm, M.; Andersson, H.; Sandberg, M.; Nilsson, H.-E. Broad-Range Hydrogel-Based pH Sensor with Capacitive Readout Manufactured on a Flexible Substrate. Chemosensors 2018, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Baki, C.N.; Akkaya, E.U. Boradiazaindacene-Appended Calix[4]arene: Fluorescence Sensing of pH Near Neutrality. J. Org. Chem. 2001, 66, 1512–1513. [Google Scholar] [CrossRef]
- Han, F.; Xu, Y.; Jiang, D.; Qin, Y.; Chen, H. A BODIPY-derived fluorescent probe for cellular pH measurements. Anal. Biochem. 2013, 435, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Liu, T.; Xu, Y.; Qin, Y. Fluorescent ion optodes based on calixarene functionized boron dipyrromethene chromoionophore for simultaneous measurement of multi-electrolytes in biological samples. Sensor. Actuat. B Chem. 2014, 192, 423–428. [Google Scholar] [CrossRef]
- Lo, P.-C.; Rodríguez-Morgade, M.S.; Pandey, R.K.; Ng, D.K.P.; Torres, T.; Dumoulin, F. The unique features and promises of phthalocyanines as advanced photosensitisers for photodynamic therapy of cancer. Chem. Soc. Rev. 2020, 49, 1041–1056. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, Y.; Nalbantoglu, T.; Durgut, T.; Akkaya, E.U. PEGylated calix[4]arene as a carrier for a BODIPY-based photosensitizer. Tetrahedron Lett. 2014, 55, 538–540. [Google Scholar] [CrossRef]
- Tosi, I.; Bardi, B.; Ambrosetti, M.; Domenichini, E.; Iagatti, A.; Baldini, L.; Cappelli, C.; Di Donato, M.; Sansone, F.; Sissa, C.; et al. Investigation of electronic energy transfer in a BODIPY-decorated calix[4]arene. Dyes Pigment. 2019, 171, 107652. [Google Scholar] [CrossRef]
- Moran, J.R.; Karbach, S.; Cram, D.J. Cavitands: Synthetic molecular vessels. J. Am. Chem. Soc. 1982, 104, 5826–5828. [Google Scholar] [CrossRef]
- Cram, D.J.; Choi, H.J.; Bryant, J.A.; Knobler, C.B. Host-guest complexation. 62. Solvophobic and entropic driving forces for forming velcraplexes, which are 4-fold, lock-key dimers in organic media. J. Am. Chem. Soc. 1992, 114, 7748–7765. [Google Scholar] [CrossRef]
- Soncini, P.; Bonsignore, S.; Dalcanale, E.; Ugozzoli, F. Cavitands as versatile molecular receptors. J. Org. Chem. 1992, 57, 4608–4612. [Google Scholar] [CrossRef]
- Roncucci, P.; Pirondini, L.; Paderni, G.; Massera, C.; Dalcanale, E.; Azov, V.A.; Diederich, F. Conformational behavior of pyrazine-bridged and mixed-bridged cavitands: A general model for solvent effects on thermal “vase-kite” switching. Chem. Eur. J. 2006, 12, 4775–4784. [Google Scholar] [CrossRef]
- Haino, T.; Rudkevich, D.M.; Shivanyuk, A.; Rissanen, K.; Rebek, J. Julius, Induced-Fit Molecular Recognition with Water-Soluble Cavitands. Chem. Eur. J. 2000, 6, 3797–3805. [Google Scholar] [CrossRef]
- Skinner, P.J.; Cheetham, A.G.; Beeby, A.; Gramlich, V.; Diederich, F. Conformational Switching of Resorcin[4]arene Cavitands by Protonation, Preliminary Communication. Helv. Chim. Acta 2001, 84, 2146–2153. [Google Scholar] [CrossRef]
- Azov, V.; Diederich, F.; Lill, Y.; Hecht, B. Synthesis and Conformational Switching of Partially and Differentially Bridged Resorcin[4]arenes Bearing Fluorescent Dye Labels Preliminary Communication. Helv. Chim. Acta 2003, 86, 2149–2155. [Google Scholar] [CrossRef]
- Azov, V.A.; Schlegel, A.; Diederich, F. Geometrically Precisely Defined Multinanometer Expansion/Contraction Motions in a Resorcin[4]arene Cavitand Based Molecular Switch. Angew. Chem. Int. Ed. 2005, 44, 4635–4638. [Google Scholar] [CrossRef] [PubMed]
- Azov, V.A.; Schlegel, A.; Diederich, F. Functionalized Calix[4]resorcinarene Cavitands. Versatile Platforms for the Modular Construction of Extended Molecular Switches. Bull. Chem. Soc. Jpn. 2006, 79, 1920–1940. [Google Scholar] [CrossRef]
- Pochorovski, I.; Breiten, B.; Schweizer, W.B.; Diederich, F. FRET Studies on a Series of BODIPY-Dye-Labeled Switchable Resorcin[4]arene Cavitands. Chem. Eur. J. 2010, 16, 12590–12602. [Google Scholar] [CrossRef] [PubMed]
- Pochorovski, I.; Knehans, T.; Nettels, D.; Müller, A.M.; Schweizer, W.B.; Caflisch, A.; Schuler, B.; Diederich, F. Experimental and Computational Study of BODIPY Dye-Labeled Cavitand Dynamics. J. Am. Chem. Soc. 2014, 136, 2441–2449. [Google Scholar] [CrossRef] [Green Version]
- Otto, J.; Wang, L.; Pochorovski, I.; Blau, S.M.; Aspuru-Guzik, A.; Bao, Z.; Engel, G.S.; Chiu, M. Disentanglement of Excited-State Dynamics with Implications for FRET Measurements: Two-Dimensional Electronic Spectroscopy of a BODIPY-Functionalized Cavitand. Chem. Sci. 2018, 9, 3694–3703. [Google Scholar] [CrossRef] [Green Version]
- Bastug, E.; Kursunlu, A.N.; Guler, E. A fluorescent clever macrocycle: Deca-BODIPY bearing a pillar[5]arene and its selective binding of asparagine in half-aqueous medium. J. Lumin. 2020, 225, 117343. [Google Scholar] [CrossRef]
- Davis, A.V.; Yeh, R.M.; Raymond, K.N. Supramolecular assembly dynamics. Proc. Natl. Acad. Sci. USA 2002, 99, 4793–4796. [Google Scholar] [CrossRef] [Green Version]
- Wehner, M.; Würthner, F. Supramolecular polymerization through kinetic pathway control and living chain growth. Nat. Rev. Chem. 2019, 4, 38–53. [Google Scholar] [CrossRef]
- Meng, L.-B.; Li, D.; Xiong, S.; Hu, X.-Y.; Wang, L.; Li, G. FRET-capable supramolecular polymers based on a BODIPY-bridged pillar[5]arene dimer with BODIPY guests for mimicking the light-harvesting system of natural photosynthesis. Chem. Commun. 2015, 51, 4643–4646. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.-B.; Zhang, W.; Li, D.; Li, Y.; Hu, X.-Y.; Wang, L.; Li, G. pH-Responsive supramolecular vesicles assembled by water-soluble pillar[5]arene and a BODIPY photosensitizer for chemo-photodynamic dual therapy. Chem. Commun. 2015, 51, 14381–14384. [Google Scholar] [CrossRef] [PubMed]
- Mena-Hernando, S.; Perez, E.M. Mechanically interlocked materials. Rotaxanes and catenanes beyond the small molecule. Chem. Soc. Rev. 2019, 48, 5016–5032. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Xiao, X.; Li, W.; Jiang, J. Multistimuli sensitive behavior of novel BODIPY-involved pillar[5]arene-based fluorescent [2]rotaxane and its supramolecular gel. Adv. Sci. 2015, 2, 1500082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aratani, N.; Kim, D.; Osuka, A. Discrete cyclic porphyrin arrays as artificial light-harvesting antenna. Acc. Chem. Res. 2009, 42, 1922–1934. [Google Scholar] [CrossRef]
- Miyatake, T.; Tamiaki, H. Self-aggregates of natural chlorophylls and their synthetic analogues in aqueous media for making light-harvesting systems. Coordin. Chem. Rev. 2010, 254, 2593–2602. [Google Scholar] [CrossRef]
- Xiao, T.; Zhong, W.; Zhou, L.; Xu, L.; Sun, X.-Q.; Elmes, R.B.P.; Hu, X.-Y.; Wang, L. Artificial light-harvesting systems fabricated by supramolecular host-guest interactions. Chin. Chem. Lett. 2019, 30, 31–36. [Google Scholar] [CrossRef]
- Kursunlu, A.N.; Baslak, C. A BODIPY-bearing pillar[5]arene for mimicking photosynthesis: Multi-fluorophoric light harvesting system. Tetrahedron Lett. 2018, 59, 1958–1962. [Google Scholar] [CrossRef]






















© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Fang, Y.; Dehaen, W. Macrocyclic Arenes Functionalized with BODIPY: Rising Stars among Chemosensors and Smart Materials. Chemosensors 2020, 8, 51. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors8030051
Huang J, Fang Y, Dehaen W. Macrocyclic Arenes Functionalized with BODIPY: Rising Stars among Chemosensors and Smart Materials. Chemosensors. 2020; 8(3):51. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors8030051
Chicago/Turabian StyleHuang, Jianjun, Yuyu Fang, and Wim Dehaen. 2020. "Macrocyclic Arenes Functionalized with BODIPY: Rising Stars among Chemosensors and Smart Materials" Chemosensors 8, no. 3: 51. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors8030051