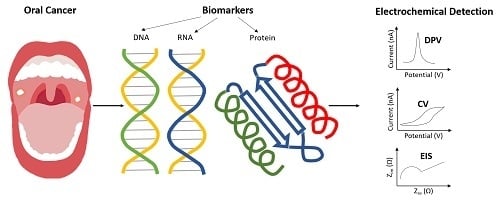
A Review: Electrochemical Biosensors for Oral Cancer
Abstract
:1. Introduction
2. Electrochemical Methodologies for Biosensing
2.1. DNA Biosensor
2.2. RNA Biosensor
2.3. Protein Biosensor
2.3.1. Proteins Biosensors Targeting IL-6 and IL-8
2.3.2. Protein Biosensors Targeting Other Biomarkers
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Montero, P.H.; Patel, S.G. Cancer of the oral cavity. Surg. Oncol. Clin. N. Am. 2015, 24, 491–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef]
- Chaturvedi, P.; Singh, A.; Chien, C.Y.; Warnakulasuriya, S. Tobacco related oral cancer. BMJ 2019, 365, l2142. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [Green Version]
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Murray, T.; Thun, M.J. Cancer statistics. CA Cancer J. Clin. 2008, 58, 71–96. [Google Scholar] [CrossRef] [PubMed]
- Messadi, D.V. Diagnostic aids for detection of oral precancerous conditions. Int. J. Oral Sci. 2013, 5, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Yen, A.M.F.; Chen, S.C.; Chen, T.H.H. Dose-response relationships of oral habits associated with the risk of oral pre-malignant lesions among men who chew betel quid. Oral Oncol. 2007, 43, 634–638. [Google Scholar] [CrossRef]
- Kumar, M.; Nanavati, R.; Modi, T.; Dobariya, C. Oral cancer: Etiology and risk factors: A review. J. Cancer Res. Ther. 2016, 12, 458–463. [Google Scholar] [CrossRef]
- Chuang, S.L.; Su, W.W.Y.; Chen, S.L.S.; Yen, A.M.F.; Wang, C.P.; Fann, J.C.Y.; Chiu, S.Y.H.; Lee, Y.C.; Chiu, H.M.; Chang, D.C.; et al. Population-based screening program for reducing oral cancer mortality in 2,334,299 taiwanese cigarette smokers and/or betel quid chewers. Cancer 2017, 123, 1597–1609. [Google Scholar] [CrossRef] [Green Version]
- Pearce, A.; Sharp, L.; Hanly, P.; Barchuk, A.; Bray, F.; de Camargo Cancela, M.; Gupta, P.; Meheus, F.; Qiao, Y.L.; Sitas, F.; et al. Productivity losses due to premature mortality from cancer in Brazil, Russia, India, China, and South Africa (BRICS): A population-based comparison. Cancer Epidemiol. 2018, 53, 27–34. [Google Scholar] [CrossRef]
- Gupta, B.; Johnson, N.W.; Kumar, N. Global epidemiology of head and neck cancers: A continuing challenge. Oncology 2016, 91, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, P.R.; Kramer, B.S.; Srivastava, S. Trends in biomarker research for cancer detection. Lancet Oncol. 2001, 2, 698–704. [Google Scholar] [CrossRef]
- Pollaers, K.; Hinton-Bayre, A.; Friedland, P.L.; Farah, C.S. AJCC 8th edition oral cavity squamous cell carcinoma staging—Is it an improvement on the AJCC 7th edition? Oral Oncol. 2018, 82, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Saadat, D.; Kwon, O.; Lee, Y.; Choi, W.S.; Kim, J.H.; Yeo, W.H. Recent advances in salivary cancer diagnostics enabled by biosensors and bioelectronics. Biosens. Bioelectron. 2016, 81, 181–197. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhang, X.Q.; Liu, Q.; Zhang, J.; Zhou, G. Nanotechnology: A promising method for oral cancer detection and diagnosis. J. Nanobiotechnol. 2018, 16, 52. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Shadjou, N.; dela Guardia, M. Non-invasive diagnosis of oral cancer: The role of electro-analytical methods and nanomaterials. TrAC Trends Anal. Chem. 2017, 91, 125–137. [Google Scholar] [CrossRef]
- Riedel, F.; Zaiss, I.; Herzog, D.; Götte, K.; Naim, R.; Hörmann, K. Serum levels of interleukin-6 in patients with primary head and neck squamous cell carcinoma. Anticancer Res. 2005, 25, 2761–2766. [Google Scholar]
- Ilkhani, H.; Sarparast, M.; Noori, A.; Bathaie, S.Z.; Mousavi, M.F. Electrochemical aptamer/antibody based sandwich immunosensor for the detection of EGFR, a cancer biomarker, using gold nanoparticles as a signaling probe. Biosens. Bioelectron. 2015, 74, 491–497. [Google Scholar] [CrossRef]
- Tan, Y.; Wei, X.; Zhao, M.; Qiu, B.; Guo, L.; Lin, Z.; Yang, H.H. Ultraselective homogeneous electrochemical biosensor for DNA species related to oral cancer based on nicking endonuclease assisted target recycling amplification. Anal. Chem. 2015, 87, 9204–9208. [Google Scholar] [CrossRef]
- Ma, R.N.; Wang, L.L.; Wang, H.F.S.; Jia, L.P.; Zhang, W.; Shang, L.; Xue, Q.W.; Jia, W.L.; Liu, Q.Y.; Wang, H.F.S. Highly sensitive ratiometric electrochemical DNA biosensor based on homogeneous exonuclease III-assisted target recycling amplification and one-step triggered dual-signal output. Sens. Actuators B Chem. 2018, 269, 173–179. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Guo, Y.; Li, J.; Fu, F.; Yang, H.H.; Chen, G. An ultrasensitive electrochemical biosensor for detection of DNA species related to oral cancer based on nuclease-assisted target recycling and amplification of DNAzyme. Chem. Commun. 2011, 47, 8004–8006. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Patel, P.; Liao, W.; Chaudhry, K.; Zhang, L.; Arellano-Garcia, M.; Hu, S.; Elashoff, D.; Zhou, H.; Shukla, S.; et al. Electrochemical sensor for multiplex biomarkers detection. Clin. Cancer Res. 2009, 15, 4446–4452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aluoch, A.O.; Sadik, O.A.; Bedi, G. Development of an oral biosensor for salivary amylase using a monodispersed silver for signal amplification. Anal. Biochem. 2005, 340, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Liao, W.; Xu, Z.; Yang, Y.; Wong, D.T.; Ho, C.M. Bio/Abiotic interface constructed from nanoscale DNA dendrimer and conducting polymer for ultrasensitive biomolecular diagnosis. Small 2009, 5, 1784–1790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malhotra, R.; Patel, V.; Vaqué, J.P.; Gutkind, J.S.; Rusling, J.F. Ultrasensitive electrochemical immunosensor for oral cancer biomarker IL-6 using carbon nanotube forest electrodes and multilabel amplification. Anal. Chem. 2010, 82, 3118–3123. [Google Scholar] [CrossRef] [Green Version]
- Saxena, S.; Sankhla, B.; Sundaragiri, K.; Bhargava, A. A review of salivary biomarker: A tool for early oral cancer diagnosis. Adv. Biomed. Res. 2017, 6, 90. [Google Scholar] [CrossRef]
- Malon, R.S.P.; Sadir, S.; Balakrishnan, M.; Córcoles, E.P. Saliva-based biosensors: Noninvasive monitoring tool for clinical diagnostics. Biomed Res. Int. 2014, 2014, 962903. [Google Scholar] [CrossRef]
- Markopoulos, A.K.; Michailidou, E.Z.; Tzimagiorgis, G. Salivary markers for oral cancer detection. Open Dent. J. 2010, 4, 172–178. [Google Scholar] [CrossRef] [Green Version]
- Torrente-Rodríguez, R.M.; Campuzano, S.; Ruiz-Valdepeñas Montiel, V.; Gamella, M.; Pingarrón, J.M. Electrochemical bioplatforms for the simultaneous determination of interleukin (IL)-8 MRNA and IL-8 protein oral cancer biomarkers in raw saliva. Biosens. Bioelectron. 2016, 77, 543–548. [Google Scholar] [CrossRef]
- Wang, Z.W.; Zhang, J.; Guo, Y.; Wu, X.Y.; Yang, W.J.; Xu, L.J.; Chen, J.H.; Fu, F.F. A novel electrically magnetic-controllable electrochemical biosensor for the ultra sensitive and specific detection of attomolar level oral cancer-related microRNA. Biosens. Bioelectron. 2013, 45, 108–113. [Google Scholar] [CrossRef]
- Otieno, B.A.; Krause, C.E.; Latus, A.; Chikkaveeraiah, B.V.; Faria, R.C.; Rusling, J.F. On-line protein capture on magnetic beads for ultrasensitive microfluidic immunoassays of cancer biomarkers. Biosens. Bioelectron. 2014, 53, 268–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Chen, R.; Xu, L.; Ning, Y.; Xie, S.; Zhang, G.J. Silicon nanowire biosensor for highly sensitive and multiplexed detection of oral squamous cell carcinoma biomarkers in saliva. Anal. Sci. 2015, 31, 73–78. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Sharma, J.G.; Maji, S.; Malhotra, B.D. Nanostructured zirconia decorated reduced graphene oxide based efficient biosensing platform for non-invasive oral cancer detection. Biosens. Bioelectron. 2016, 78, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, S.; Tiwari, S.; Augustine, S.; Srivastava, S.; Yadav, B.K.; Malhotra, B.D. Highly sensitive protein functionalized nanostructured hafnium oxide based biosensing platform for non-invasive oral cancer detection. Sens. Actuators B Chem. 2016, 235, 1–10. [Google Scholar] [CrossRef]
- Qureshi, A.; Gurbuz, Y.; Niazi, J.H. Label-free capacitance based aptasensor platform for the detection of HER2/ErbB2 cancer biomarker in serum. Sens. Actuators B Chem. 2015, 220, 1145–1151. [Google Scholar] [CrossRef]
- Lin, T.E.; Chen, W.H.; Shiang, Y.C.; Huang, C.C.; Chang, H.T. Colorimetric detection of platelet-derived growth factors through competitive interactions between proteins and functional gold nanoparticles. Biosens. Bioelectron. 2011, 29, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Godolphin, W.; Jones, L.A.; Holt, J.A.; Wong, S.G.; Keith, D.E.; Levin, W.J.; Stuart, S.G.; Udove, J.; Ullrich, A.; et al. Studies of the HER-2/Neu proto-oncogene in human breast and ovarian cancer. Science 1989, 244, 707–712. [Google Scholar] [CrossRef]
- Kumar, L.S.S.; Wang, X.; Hagen, J.; Naik, R.; Papautsky, I.; Heikenfeld, J. Label free nano-aptasensor for interleukin-6 in protein-dilute bio fluids such as sweat. Anal. Methods 2016, 8, 3440–3444. [Google Scholar] [CrossRef]
- Tertis, M.; Leva, P.I.; Bogdan, D.; Suciu, M.; Graur, F.; Cristea, C. Impedimetric aptasensor for the label-free and selective detection of interleukin-6 for colorectal cancer screening. Biosens. Bioelectron. 2019, 137, 123–132. [Google Scholar] [CrossRef]
- Thomas, J.H.; Kim, S.K.; Hesketh, P.J.; Halsall, H.B.; Heineman, W.R. Microbead-based electrochemical immunoassay with interdigitated array electrodes. Anal. Biochem. 2004, 328, 113–122. [Google Scholar] [CrossRef]
- Li, H.; Wei, Q.; He, J.; Li, T.; Zhao, Y.; Cai, Y.; Du, B.; Qian, Z.; Yang, M. Electrochemical immunosensors for cancer biomarker with signal amplification based on ferrocene functionalized iron oxide nanoparticles. Biosens. Bioelectron. 2011, 26, 3590–3595. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.A.; Schnell, F.; Kaveh-Baghbaderani, Y.; Berensmeier, S.; Schwaminger, S.P. Immunomagnetic separation of microorganisms with iron oxide nanoparticles. Chemosensors 2020, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Evtugyn, G.; Hianik, T. Electrochemical immuno- and aptasensors for mycotoxin determination. Chemosensors 2019, 7, 10. [Google Scholar] [CrossRef] [Green Version]
- Vasilescu, A.; Fanjul-Bolado, P.; Titoiu, A.M.; Porumb, R.; Epure, P. Progress in electrochemical (bio)sensors for monitoring wine production. Chemosensors 2019, 7, 66. [Google Scholar] [CrossRef] [Green Version]
- Shellaiah, M.; Sun, K.W. Review on nanomaterial-based melamine detection. Chemosensors 2019, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Zakaria, A.B.M.; Leszczynska, D. Electrochemically prepared unzipped single walled carbon nanotubes-MnO2 nanostructure composites for hydrogen peroxide and glucose sensing. Chemosensors 2019, 7, 1. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, S.; Gupta, P.K.; Bagbi, Y.; Sarkar, T.; Solanki, P.R. L-Cysteine capped lanthanum hydroxide nanostructures for non-invasive detection of oral cancer biomarker. Biosens. Bioelectron. 2017, 89, 1042–1052. [Google Scholar] [CrossRef]
- Choudhary, M.; Yadav, P.; Singh, A.; Kaur, S.; Ramirez-Vick, J.; Chandra, P.; Arora, K.; Singh, S.P. CD 59 targeted ultrasensitive electrochemical immunosensor for fast and noninvasive diagnosis of oral cancer. Electroanalysis 2016, 28, 2565–2574. [Google Scholar] [CrossRef]
- Verma, S.; Singh, A.; Shukla, A.; Kaswan, J.; Arora, K.; Ramirez-Vick, J.; Singh, P.; Singh, S.P. Anti-IL8/AuNPs-RGO/ITO as an immunosensing platform for noninvasive electrochemical detection of oral Cancer. ACS Appl. Mater. Interfaces 2017, 9, 27462–27474. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, A.; Aghaie, A.; Vahedi, E.; Qazvini, A.; Ghanei, M.; Afkhami, A.; Hajian, A.; Bagheri, H. Electrochemical biosensors for the detection of lung cancer biomarkers: A review. Talanta 2020, 206, 120251. [Google Scholar] [CrossRef]
- Darvishi, S.; Pick, H.; Lin, T.E.; Zhu, Y.; Li, X.; Ho, P.C.; Girault, H.H.; Lesch, A. Tape-stripping electrochemical detection of melanoma. Anal. Chem. 2019, 91, 12900–12908. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-E.; Lu, Y.-J.; Sun, C.-L.; Pick, H.; Chen, J.-P.; Lesch, A.; Girault, H.H. Soft electrochemical probes for mapping the distribution of biomarkers and injected nanomaterials in animal and human tissues. Angew. Chem. Int. Ed. 2017, 56, 16498–16502. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.E.; Bondarenko, A.; Lesch, A.; Pick, H.; Cortés-Salazar, F.; Girault, H.H. Monitoring tyrosinase expression in non-metastatic and metastatic melanoma tissues by scanning electrochemical microscopy. Angew. Chem. Int. Ed. 2016, 55, 3813–3816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pachauri, N.; Dave, K.; Dinda, A.; Solanki, P.R. Cubic CeO2 implanted reduced graphene oxide-based highly sensitive biosensor for non-invasive oral cancer biomarker detection. J. Mater. Chem. B 2018, 6, 3000–3012. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Shadjou, N.; dela Guardia, M. Early stage screening of breast cancer using electrochemical biomarker detection. TrAC Trends Anal. Chem. 2017, 91, 67–76. [Google Scholar] [CrossRef]
- Singh, S.; Gill, A.A.S.; Nlooto, M.; Karpoormath, R. Prostate cancer biomarkers detection using nanoparticles based electrochemical biosensors. Biosens. Bioelectron. 2019, 137, 213–221. [Google Scholar] [CrossRef]
- Ding, S.; Das, S.R.; Brownlee, B.J.; Parate, K.; Davis, T.M.; Stromberg, L.R.; Chan, E.K.L.; Katz, J.; Iverson, B.D.; Claussen, J.C. CIP2A immunosensor comprised of vertically-aligned carbon nanotube interdigitated electrodes towards point-of-care oral cancer screening. Biosens. Bioelectron. 2018, 117, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Aydın, M.; Aydın, E.B.; Sezgintürk, M.K. A highly selective electrochemical immunosensor based on conductive carbon black and star PGMA polymer composite material for IL-8 biomarker detection in human serum and saliva. Biosens. Bioelectron. 2018, 117, 720–728. [Google Scholar] [CrossRef]
- Verma, S.; Singh, S.P. Non-invasive oral cancer detection from saliva using Zinc oxide-reduced graphene oxide nanocomposite based bioelectrode. MRS Commun. 2019, 9, 1227–1234. [Google Scholar] [CrossRef]
- Deckert, F.; Legay, F. Development and validation of an IL-6 immuno-receptor assay based on surface plasmon resonance. J. Pharm. Biomed. Anal. 2000, 23, 403–411. [Google Scholar] [CrossRef]
- Malhotra, R.; Urs, A.B.; Chakravarti, A.; Kumar, S.; Gupta, V.K.; Mahajan, B. Correlation of cyfra 21-1 Levels in saliva and serum with CK19 MRNA expression in oral squamous cell carcinoma. Tumor Biol. 2016, 37, 9263–9271. [Google Scholar] [CrossRef] [PubMed]
- Alevizos, I.; Mahadevappa, M.; Zhang, X.; Ohyama, H.; Kohno, Y.; Posner, M.; Gallagher, G.T.; Varvares, M.; Cohen, D.; Kim, D.; et al. Oral cancer in vivo gene expression profiling assisted by laser capture microdissection and microarray analysis. Oncogene 2001, 20, 6196–6204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babiuch, K.; Kuśnierz-Cabala, B.; Kęsek, B.; Okoń, K.; Darczuk, D.; Chomyszyn-Gajewska, M. Evaluation of proinflammatory, NF-KappaB dependent cytokines: IL-1α, IL-6, IL-8, and TNF-α in tissue specimens and saliva of patients with oral squamous cell carcinoma and oral potentially malignant disorders. J. Clin. Med. 2020, 9, 867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahibzada, H.A.; Khurshid, Z.; Khan, R.S.; Naseem, M.; Siddique, K.M.; Mali, M.; Zafar, M.S. Salivary IL-8, IL-6 and TNF-α as potential diagnostic biomarkers for oral cancer. Diagnostics 2017, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Malhotra, P.S.; Thomas, G.R.; Ondrey, F.G.; Duffey, D.C.; Smith, C.W.; Enamorado, I.; Yeh, N.T.; Kroog, G.S.; Rudy, S.; et al. Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin. Cancer Res. 1999, 5, 1369–1379. [Google Scholar] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Il-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Gokhale, A.S.; Haddad, R.I.; Cavacini, L.A.; Wirth, L.; Weeks, L.; Hallar, M.; Faucher, J.; Posner, M.R. Serum concentrations of interleukin-8, vascular endothelial growth factor, and epidermal growth factor receptor in patients with squamous cell cancer of the head and neck. Oral Oncol. 2005, 41, 70–76. [Google Scholar] [CrossRef]
- Chen, X.; Jia, X.; Han, J.; Ma, J.; Ma, Z. Electrochemical immunosensor for simultaneous detection of multiplex cancer biomarkers based on graphene nanocomposites. Biosens. Bioelectron. 2013, 50, 356–361. [Google Scholar] [CrossRef]
- Wei, Q.; Mao, K.; Wu, D.; Dai, Y.; Yang, J.; Du, B.; Yang, M.; Li, H. A novel label-free electrochemical immunosensor based on graphene and thionine nanocomposite. Sens. Actuators B Chem. 2010, 149, 314–318. [Google Scholar] [CrossRef]
- Darvishi, S.; Souissi, M.; Kharaziha, M.; Karimzadeh, F.; Sahara, R.; Ahadian, S. Gelatin methacryloyl hydrogel for glucose biosensing using ni nanoparticles-reduced graphene oxide: An experimental and modeling study. Electrochim. Acta 2018, 261, 275–283. [Google Scholar] [CrossRef]
- Darvishi, S.; Souissi, M.; Karimzadeh, F.; Kharaziha, M.; Sahara, R.; Ahadian, S. Ni nanoparticle-decorated reduced graphene oxide for non-enzymatic glucose sensing: An experimental and modeling study. Electrochim. Acta 2017, 240, 388–398. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Khanra, P.; Mishra, A.K.; Kim, N.H.; Lee, J.H. Recent advances in graphene-based biosensors. Biosens. Bioelectron. 2011, 26, 4637–4648. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.P.; Zhu, H.G.; Zhang, C.P.; Chen, W.T.; Zhang, Z.Y. Detection of serum cyfra 21-1 in patients with primary oral squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2007, 36, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.D.; Allen, M.J.; Tung, V.C.; Yang, Y.; Kaner, R.B.; Weiller, B.H. Practical chemical sensors from chemically derived graphene. ACS Nano 2009, 3, 301–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Batchelor-McAuley, C.; Shao, L.; Sokolov, S.V.; Young, N.P.; Compton, R.G. Quantifying single-carbon nanotube-electrode contact via the nanoimpact method. J. Phys. Chem. Lett. 2017, 8, 507–511. [Google Scholar] [CrossRef]
- Böckelman, C.; Hagström, J.; Mäkinen, L.K.; Keski-Säntti, H.; Häyry, V.; Lundin, J.; Atula, T.; Ristimäki, A.; Haglund, C. High CIP2A immunoreactivity is an independent prognostic indicator in early-stage tongue cancer. Br. J. Cancer 2011, 104, 1890–1895. [Google Scholar] [CrossRef] [Green Version]





| Biomarker | Sample | Electrochemical Method | Detection Limits | The Levels of The Biomarker in Normal Case and Cancer Patient | References |
|---|---|---|---|---|---|
| Amylase | Samples spiked in potassium ferrocyanide | Cyclic voltammetry | 1.57 pg mL−1 | - | [23] |
| IL-8 protein, IL-1β protein and IL-8 mRNA | Samples spiked in buffer | cyclic square-waveform | Protein:100–200 fg mL−1 mRNA IL-8:10 aM | IL-8 protein, OSCC patient: 720 pg mL−1; Normal: 250 pg mL−1 IL-8 mRNA, patient: 16 fM; Normal:2 fM | [24] |
| Interleukin-6 (IL-6) | HNSCC cell lines | Amperometry | 2.5 × 10−14 M | IL-6 protein, HNSCC patient: more than 20 pg mL−1; Normal: less than 6 pg mL−1 | [25] |
| microRNA | Artificial saliva | Cyclic voltammetry Chronoamperometry | 2.2 × 10−19 M | - | [30] |
| IL-6 protein, IL-8 protein | Serum | Amperometry | IL-6:5 fg mL−1, IL-8:7 fg mL−1 | IL-6 protein, HNSCC patient: more than 20 pgmL−1; Normal: less than 6 pg ml−1 IL-8 protein, patient: 720 pg mL−1; Normal: 250 pg mL−1 | [31] |
| Oral Cancer Overexpressed 1 | Human saliva | Differential pulse voltammetry | 0.35 pM | - | [19] |
| IL-8 protein, TNF-α | Artificial saliva | I-V Curve | 100 fg mL-1 | IL-8 protein, patient: 720 pg mL−1; Normal: 250 pg mL−1 | [32] |
| IL-8 mRNA IL-8 protein | Human saliva | Amperometry | IL-8 mRNA: 0.21 nM IL-8: 72.4 pg mL−1 | IL-8 mRNA, patient: 16 fM; Normal:2 fM IL-8 protein, patient: 720 pg mL−1; Normal: 250 pg mL−1 | [29] |
| CYFRA-21-1 | Samples spiked in PBS buffer | Cyclic voltammetry Electrochemical impedance spectroscopy | 0.21 ng mL−1 (calculated) | CYFRA-21-1 protein, normal: 3.8 ng mL−1; patient:17.46 ± 1.46 ng mL−1 | [34] |
| CYFRA-21-1 | Samples spiked in PBS buffer | Cyclic voltammetry Differential pulse voltammetry | 0.122 ng mL−1 (calculated) | CYFRA-21-1 protein, normal: 3.8 ng mL−1; Patient:17.46 ± 1.46 ng mL−1 | [33] |
| CYFRA-21-1 | Artificial Saliva | Differential pulse voltammetry | 0.001 ng mL−1 | CYFRA-21-1 protein, normal: 3.8 ng mL−1; Patient:17.46 ± 1.46 ng mL−1 | [47] |
| CD59 | Human saliva | Cyclic voltammetry Electrochemical impedance spectroscopy | Treated Saliva: 0.84 ± 0.04 fg mL−1 Raw saliva: 1.46 ± 0.05 fg mL−1 | - | [48] |
| IL-8 protein | Human saliva | Cyclic voltammetry Differential pulse voltammetry | 72.73 ± 0.18 pg mL−1 (calculated) | IL-8 protein, patient: 720 pg mL−1; normal: 250 pg mL−1 | [49] |
| Oral Cancer Overexpressed 1 | Artificial saliva | Alternating current voltammetric Electrochemical impedance spectroscopy | 12.8 fM | - | [20] |
| CIP2A | Human saliva | Cyclic voltammetry Electrochemical impedance spectroscopy | 0.24 pg mL−1 | - | [57] |
| CYFRA-21-1 | Human saliva | Differential pulse voltammetry Electrochemical impedance spectroscopy | 0.625 pg mL−1 | CYFRA-21-1 protein, normal: 3.8 ng mL−1; Patients: 17.46 ± 1.46 ng mL−1 | [54] |
| IL-8 protein | Human serum and saliva | Cyclic voltammetry Electrochemical impedance spectroscopy Single Frequency Impedance | 3.3 fg mL−1 | IL-8 protein, patient: 720 pg mL−1; Normal: 250 pg mL−1 | [58] |
| IL-8 protein | Human saliva | Cyclic voltammetry Differential pulse voltammetry | 51.53 ± 0.43 pg mL−1 (calculated) | IL-8 protein, patient: 720 pg mL−1; Normal: 250 pg mL−1 | [59] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-T.; Darvishi, S.; Preet, A.; Huang, T.-Y.; Lin, S.-H.; Girault, H.H.; Wang, L.; Lin, T.-E. A Review: Electrochemical Biosensors for Oral Cancer. Chemosensors 2020, 8, 54. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors8030054
Lin Y-T, Darvishi S, Preet A, Huang T-Y, Lin S-H, Girault HH, Wang L, Lin T-E. A Review: Electrochemical Biosensors for Oral Cancer. Chemosensors. 2020; 8(3):54. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors8030054
Chicago/Turabian StyleLin, Yen-Tzu, Sorour Darvishi, Anant Preet, Tzu-Yen Huang, Sheng-Hsuan Lin, Hubert H. Girault, Ligang Wang, and Tzu-En Lin. 2020. "A Review: Electrochemical Biosensors for Oral Cancer" Chemosensors 8, no. 3: 54. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors8030054