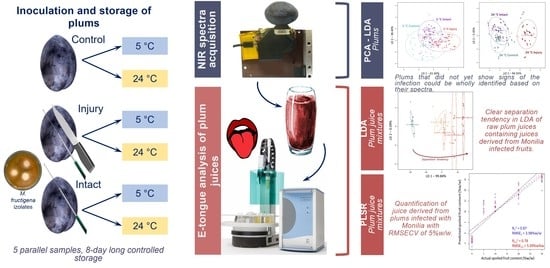
Detection of Monilia Contamination in Plum and Plum Juice with NIR Spectroscopy and Electronic Tongue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fruit Samples and Fungal Isolates
2.2. Sample Preparation
2.3. Methods
2.3.1. Spectral Acquisition of the Plum Samples with Hand-Held Spectrometer
2.3.2. Electronic Tongue Analysis of the Plum Juice Samples
2.3.3. Data Analysis
Multivariate Analysis of the NIR Spectra
Multivariate Analysis of the E-Tongue Data
3. Results and Discussion
3.1. Results of Near Infrared Spectroscopy
3.1.1. Discrimination of the Different Treatment Groups of the Plum Samples with the Hand-Held Spectrometer
3.1.2. Early Detection of Monilia fructigena Contamination on Plums with the Hand-Held Spectrometer
3.2. Results of Electronic Tongue
3.2.1. Discrimination of the Different Treatment Groups of the Plum Samples with E-Tongue
3.2.2. Detection and Quantification of Spoiled Fruit Content in Raw Plum Juices with E-Tongue
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT Plums and Sloes. Available online: http://data.un.org/Data.aspx?d=FAO&f=itemCode%3A536 (accessed on 22 September 2021).
- Mühlbauer, W.; Müller, J. Chapter 9.11—Plum (Prunus Domestica Subsp. Domestica). In Drying Atlas; Mühlbauer, W., Müller, J.B.T.-D.A., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 355–363. ISBN 978-0-12-818162-1. [Google Scholar]
- Birwal, P.; Deshmukh, G.; Saurabh, S. Plums: A Brief Introduction. J. Food Nutr. Popul. Heal. 2017, 1, 1–5. [Google Scholar]
- Sinha, N.K. Handbook of Fruits and Fruit Processing, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; ISBN 9780813808949. [Google Scholar]
- Padayachee, A.; Day, L.; Howell, K.; Gidley, M.J. Complexity and health functionality of plant cell wall fibers from fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2017, 57, 59–81. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Lian, Y.; Zhao, C.; Du, H.; Han, Y.; Gao, W.; Xiao, H.; Zheng, J. Dietary fibers from fruits and vegetables and their health benefits via modulation of gut microbiota. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1514–1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lara, M.V.; Bonghi, C.; Famiani, F.; Vizzotto, G.; Walker, R.P.; Drincovich, M.F. Stone fruit as biofactories of phytochemicals with potential roles in human nutrition and health. Front. Plant Sci. 2020, 11, 1323. [Google Scholar] [CrossRef]
- Igwe, E.O.; Charlton, K.E. A systematic review on the health effects of plums (Prunus domestica and Prunus salicina). Phyther. Res. 2016, 30, 701–731. [Google Scholar] [CrossRef]
- Topp, B.L.; Russell, D.M.; Neumüller, M.; Dalbó, M.A.; Liu, W. Plum. In Fruit Breeding; Springer: Berlin, Germany, 2012; pp. 571–621. [Google Scholar]
- Miranda-Castro, S.P. Chapter 3—Application of Chitosan in Fresh and Minimally Processed Fruits and Vegetables. In Chitosan in the Preservation of Agricult Application of Chitosan in Fresh and Minimally Processed Fruits and Vegetablesural Commodities; Academic Press: Cambridge, MA, USA, 2016; pp. 67–113. ISBN 9780128027356. [Google Scholar]
- Elik, A.; Yanik, D.K.; Istanbullu, Y.; Guzelsoy, N.A.; Yavuz, A.; Gogus, F. Strategies to reduce post-harvest losses for fruits and vegetables. Strategies 2019, 5, 29–39. [Google Scholar]
- Aiello, D.; Restuccia, C.; Stefani, E.; Vitale, A.; Cirvilleri, G. Postharvest biocontrol ability of Pseudomonas synxantha against Monilinia fructicola and Monilinia fructigena on stone fruit. Postharvest Biol. Technol. 2019, 149, 83–89. [Google Scholar] [CrossRef]
- Singh, D.; Sharma, R.R. Postharvest Diseases of Fruits and Vegetables and Their Management; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Oliveira Lino, L.; Pacheco, I.; Mercier, V.; Faoro, F.; Bassi, D.; Bornard, I.; Quilot-Turion, B. Brown Rot Strikes Prunus Fruit: An Ancient Fight Almost Always Lost. J. Agric. Food Chem. 2016, 64, 4029–4047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petróczy, M.; Szigethy, A.; Palkovics, L. Monilinia species in Hungary: Morphology, culture characteristics, and molecular analysis. Trees Struct. Funct. 2012, 26, 153–164. [Google Scholar] [CrossRef]
- Barkai-Golan, R. Postharvest Diseases of Fruits and Vegetables: Development and Control; Elsevier: Amsterdam, The Netherlands, 2001; ISBN 0080539297. [Google Scholar]
- Xie, L.; Wang, A.; Xu, H.; Fu, X.; Ying, Y. Applications of near-infrared systems for quality evaluation of fruits: A review. Trans. ASABE 2016, 59, 399–419. [Google Scholar]
- Aouadi, B.; Zaukuu, J.-L.Z.; Vitális, F.; Bodor, Z.; Fehér, O.; Gillay, Z.; Bazar, G.; Kovacs, Z. Historical evolution and food control achievements of near infrared spectroscopy, electronic nose, and electronic tongue—Critical overview. Sensors 2020, 20, 5479. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, I.; Panigrahi, S.S.; Ravikanth, L.; Singh, C.B. Potential of Near-Infrared (NIR) Spectroscopy and Hyperspectral Imaging for Quality and Safety Assessment of Fruits: An Overview. Food Anal. Methods 2019, 12, 2438–2458. [Google Scholar] [CrossRef]
- Li, M.; Lv, W.; Zhao, R.; Guo, H.; Liu, J.; Han, D. Non-destructive assessment of quality parameters in ‘Friar’ plums during low temperature storage using visible/near infrared spectroscopy. Food Control 2017, 73, 1334–1341. [Google Scholar] [CrossRef]
- Pérez-Marín, D.; Paz, P.; Guerrero, J.-E.; Garrido-Varo, A.; Sánchez, M.-T. Miniature handheld NIR sensor for the on-site non-destructive assessment of post-harvest quality and refrigerated storage behavior in plums. J. Food Eng. 2010, 99, 294–302. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Wang, Y.W.; Gong, D.J.; Niu, X.Y.; Cheng, W.; Gu, Y.H. Discrimination of plum browning with near infrared spectroscopy. Guang Pu Xue Yu Guang Pu Fen Xi 2016, 36, 2089–2093. [Google Scholar] [PubMed]
- Siedliska, A.; Baranowski, P.; Zubik, M.; Mazurek, W.; Sosnowska, B. Detection of fungal infections in strawberry fruit by VNIR/SWIR hyperspectral imaging. Postharvest Biol. Technol. 2018, 139, 115–126. [Google Scholar] [CrossRef]
- Folch-Fortuny, A.; Prats-Montalbán, J.M.; Cubero, S.; Blasco, J.; Ferrer, A. VIS/NIR hyperspectral imaging and N-way PLS-DA models for detection of decay lesions in citrus fruits. Chemom. Intell. Lab. Syst. 2016, 156, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Zhou, D.; Tu, S.; Xiao, H.; Zhang, B.; Sun, Y.; Pan, L.; Tu, K. Quantitative visualization of fungal contamination in peach fruit using hyperspectral imaging. Food Anal. Methods 2020, 13, 1262–1270. [Google Scholar] [CrossRef]
- Everstine, K.; Spink, J.; Kennedy, S. Economically Motivated Adulteration (EMA) of Food: Common Characteristics of EMA Incidents. J. Food Prot. 2013, 76, 723–735. [Google Scholar] [CrossRef]
- Xu, L.; Xu, Z.; Liao, X. A review of fruit juice authenticity assessments: Targeted and untargeted analyses. Crit. Rev. Food Sci. Nutr. 2021, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.I.; Brewster, V.L.; Dunn, W.B.; Allwood, J.W.; Golovanov, A.P.; Goodacre, R. Fingerprinting food: Current technologies for the detection of food adulteration and contamination. Chem. Soc. Rev. 2012, 41, 5706–5727. [Google Scholar] [CrossRef] [PubMed]
- Peris, M.; Escuder-Gilabert, L. Electronic noses and tongues to assess food authenticity and adulteration. Trends Food Sci. Technol. 2016, 58, 40–54. [Google Scholar] [CrossRef] [Green Version]
- Bengtsson, G.B.; Lundby, F.; Haugen, J.-E. Rapid analytical techniques for measurement of plum ripeness postharvest. In Proceedings of the VIII International Symposium on Plum and Prune Genetics, Breeding and Pomology 734, Lofthus, Norway, 5 September 2004; pp. 211–216. [Google Scholar]
- Rudnitskaya, A.; Legin, A.; Makarychev-Mikhailov, S.; Goryacheva, O.; Vlasov, Y. Quality monitoring of fruit juices using an electronic tongue. Anal. Sci. 2002, 17, i309–i312. [Google Scholar]
- Hong, X.; Wang, J.; Qiu, S. Authenticating cherry tomato juices—Discussion of different data standardization and fusion approaches based on electronic nose and tongue. Food Res. Int. 2014, 60, 173–179. [Google Scholar] [CrossRef]
- Hong, X.; Wang, J. Detection of adulteration in cherry tomato juices based on electronic nose and tongue: Comparison of different data fusion approaches. J. Food Eng. 2014, 126, 89–97. [Google Scholar] [CrossRef]
- Wang, H.; Sun, H. Potential use of electronic tongue coupled with chemometrics analysis for early detection of the spoilage of Zygosaccharomyces rouxii in apple juice. Food Chem. 2019, 290, 152–158. [Google Scholar] [CrossRef]
- Poniatowska, A.; Michalecka, M.; Bielenin, A. Characteristic of Monilinia spp. fungi causing brown rot of pome and stone fruits in Poland. Eur. J. Plant Pathol. 2013, 135, 855–865. [Google Scholar] [CrossRef]
- NCBI Basic Local Alignment Search Tool. Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 16 September 2021).
- Alpha, M.O.S. αAstree Electronic Tongue User Manual. Available online: https://www.alpha-mos.com/astree-taste-analysis (accessed on 5 July 2020).
- Kovács, Z. Módszer Elektronikus Nyelvvel Végzett Méréseknél Fellépő Zavaró Hatások Csökkentésére = Method to Decrease the Disturbing Effects Occurring by the Electronic Tongue Measurement; Budapesti Corvinus Egyetem: Budapest, Hungary, 2012. [Google Scholar]
- Kovacs, Z.; Szöllosi, D.; Zaukuu, J.-L.Z.; Bodor, Z.; Vitális, F.; Aouadi, B.; Zsom-Muha, V.; Gillay, Z. Factors influencing the long-term stability of electronic tongue and application of improved drift correction methods. Biosensors 2020, 10, 74. [Google Scholar] [CrossRef] [PubMed]
- de Morais, T.C.B.; Rodrigues, D.R.; de Carvalho Polari Souto, U.T.; Lemos, S.G. A simple voltammetric electronic tongue for the analysis of coffee adulterations. Food Chem. 2019, 273, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, Z.; Pollner, B. Aquaphotomics-Software R-Package “aquap2“. In Proceedings of the Understanding Water in Biology 2nd International Symposium, Kobe, Japan, 26–29 November 2016; pp. 26–29. [Google Scholar]







| Sample Name | Sample Count | Mode of Inoculation |
|---|---|---|
| Control | 2 × 5 | There was no infection. |
| Injury | 2 × 5 | A cut of about −1 cm was applied to the fruit surface with a sterile knife tip. The plums were infected via this wound with culture medium edge interlaced with fungal mycelia by using sterile inoculation loops. |
| Intact | 2 × 5 | The sound fruit surface was inoculated in a circle about 1 cm in diameter with culture medium edge interlaced with fungal mycelia by using sterile inoculation loops. |
| Accuracy | % | 24 °C Control | 24 °C Injury | 24 °C Intact | 5 °C Control | 5 °C Injury | 5 °C Intact | Correct Classification |
|---|---|---|---|---|---|---|---|---|
| Recognition N = 289 | 24 °C Control | 67.39 | 5.26 | 22.45 | 0 | 6.67 | 0 | 72.29% |
| 24 °C Injury | 6.52 | 94.74 | 0 | 0 | 0 | 0 | ||
| 24 °C Intact | 17.39 | 0 | 77.55 | 10.42 | 0 | 0 | ||
| 5 °C Control | 0 | 0 | 0 | 50.00 | 26.67 | 16.33 | ||
| 5 °C Injury | 8.7 | 0 | 0 | 12.50 | 64.44 | 4.08 | ||
| 5 °C Intact | 0 | 0 | 0 | 27.08 | 2.22 | 79.59 | ||
| Validation N = 68 | 24 °C Control | 100 | 0 | 33.33 | 0 | 20.00 | 13.33 | 56.67% |
| 24 °C Injury | 0 | 100 | 0 | 0 | 0 | 0 | ||
| 24 °C Intact | 0 | 0 | 33.33 | 0 | 0 | 0 | ||
| 5 °C Control | 0 | 0 | 0 | 40.00 | 40.00 | 40.00 | ||
| 5 °C Injury | 0 | 0 | 0 | 0 | 40.00 | 20.00 | ||
| 5 °C Intact | 0 | 0 | 33.33 | 60 | 0 | 26.67 |
| Accuracy | % | 5 °C Control | 5 °C Injury | 5 °C Intact | Correct Classification |
|---|---|---|---|---|---|
| Recognition N = 144 | 5 °C Control | 68.29 | 7.41 | 12.24 | 74.41% |
| 5 °C Injury | 9.76 | 81.48 | 14.29 | ||
| 5 °C Intact | 21.95 | 11.11 | 73.47 | ||
| Validation N = 33 | 5 °C Control | 0 | 50.00 | 0 | 43.33% |
| 5 °C Injury | 8.33 | 50.00 | 20.00 | ||
| 5 °C Intact | 91.67 | 0 | 80.00 |
| Accuracy | % | 24 °C Control | 24 °C Injury | 24 °C Intact | Correct Classification |
|---|---|---|---|---|---|
| Recognition N = 145 | 24 °C Control | 93.02 | 5.56 | 31.82 | 85.21% |
| 24 °C Injury | 0 | 94.44 | 0 | ||
| 24 °C Intact | 6.98 | 0 | 68.18 | ||
| Validation N = 35 | 24 °C Control | 23.08 | 0 | 14.29 | 66.82% |
| 24 °C Injury | 23.08 | 91.67 | 0 | ||
| 24 °C Intact | 53.85 | 8.33 | 85.71 |
| Sample Sets | Storage Day | Plum 1. | Plum 2. | Plum 3. | Plum 4. | Plum 5. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vis | NIR | Vis | NIR | Vis | NIR | Vis | NIR | Vis | NIR | ||
| Independent prediction set | Day 1. | ― | + * | ― | + * | ― | + * | ― | + * | ― | + * |
| Day 2. | ― | + * | ― | + * | ― | + * | ― | + * | ― | + * | |
| Model building set | Day 3. | + | + | + | ― | ― | |||||
| Day 4. | + | + | + | ― | ― | ||||||
| Day 5. | + | + | + | ― | + | ||||||
| Day 6. | + | + | + | + | + | ||||||
| Day 7. | + | + | + | + | + | ||||||
| Day 8. | + | + | + | + | + | ||||||
| Accuracy | 24 °C Control | 24 °C Injury | 24 °C Intact | 5 °C Control | 5 °C Injury | 5 °C Intact | Correct Classification | |
|---|---|---|---|---|---|---|---|---|
| Recognition N = 35 | 24 °C Control | 83.33 | 0 | 0 | 16.67 | 16.67 | 0 | 88.89% |
| 24 °C Injury | 0 | 100 | 0 | 0 | 0 | 0 | ||
| 24 °C Intact | 0 | 0 | 100 | 0 | 0 | 0 | ||
| 5 °C Control | 0 | 0 | 0 | 66.67 | 0 | 0 | ||
| 5 °C Injury | 16.67 | 0 | 0 | 16.67 | 83.33 | 0 | ||
| 5 °C Intact | 0 | 0 | 0 | 0 | 0 | 100 | ||
| Validation N = 16 | 24 °C Control | 66.67 | 0 | 33.33 | 0 | 0 | 0 | 63.89% |
| 24 °C Injury | 0 | 100 | 0 | 0 | 0 | 50.00 | ||
| 24 °C Intact | 0 | 0 | 66.67 | 0 | 0 | 0 | ||
| 5 °C Control | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 5 °C Injury | 33.33 | 0 | 0 | 50.00 | 100 | 0 | ||
| 5 °C Intact | 0 | 0 | 0 | 50.00 | 0 | 50.00 |
| Accuracy | 5 °C Control | 5 °C Injury | 5 °C Intact | Correct Classification | |
|---|---|---|---|---|---|
| Recognition N = 18 | 5 °C Control | 100 | 0 | 0 | 100% |
| 5 °C Injury | 0 | 100 | 0 | ||
| 5 °C Intact | 0 | 0 | 100 | ||
| Validation N = 7 | 5 °C Control | 0 | 0 | 0 | 66.67% |
| 5 °C Injury | 50 | 100 | 0 | ||
| 5 °C Intact | 50 | 0 | 100 |
| Accuracy | 24 °C Control | 24 °C Injury | 24 °C Intact | Correct Classification | |
|---|---|---|---|---|---|
| Recognition N = 18 | 24 °C Control | 100 | 0 | 0 | 100% |
| 24 °C Injury | 0 | 100 | 0 | ||
| 24 °C Intact | 0 | 0 | 100 | ||
| Validation N = 8 | 24 °C Control | 100 | 0 | 0 | 100% |
| 24 °C Injury | 0 | 100 | 0 | ||
| 24 °C Intact | 0 | 0 | 100 |
| Accuracy | 24 °C Control | 24 °C Control + Injury 5% | 24 °C Control + Injury 10% | 24 °C Control + Injury 20% | 24 °C Control + Injury 30% | Correct Classification | |
|---|---|---|---|---|---|---|---|
| Recognition N = 25 | 24 °C Control | 100 | 0 | 0 | 0 | 0 | 100% |
| 24 °C Control + Injury 5% | 0 | 100 | 0 | 0 | 0 | ||
| 24 °C Control + Injury 10% | 0 | 0 | 100 | 0 | 0 | ||
| 24 °C Control + Injury 20% | 0 | 0 | 0 | 100 | 0 | ||
| 24 °C Control + Injury 30% | 0 | 0 | 0 | 0 | 100 | ||
| Validation N = 14 | 24 °C Control | 100 | 0 | 0 | 0 | 0 | 86.67% |
| 24 °C Control + Injury 5% | 0 | 100 | 0 | 0 | 0 | ||
| 24 °C Control + Injury 10% | 0 | 0 | 33.33 | 0 | 0 | ||
| 24 °C Control + Injury 20% | 0 | 0 | 66.67 | 100 | 0 | ||
| 24 °C Control + Injury 30% | 0 | 0 | 0 | 0 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitalis, F.; Tjandra Nugraha, D.; Aouadi, B.; Aguinaga Bósquez, J.P.; Bodor, Z.; Zaukuu, J.-L.Z.; Kocsis, T.; Zsom-Muha, V.; Gillay, Z.; Kovacs, Z. Detection of Monilia Contamination in Plum and Plum Juice with NIR Spectroscopy and Electronic Tongue. Chemosensors 2021, 9, 355. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors9120355
Vitalis F, Tjandra Nugraha D, Aouadi B, Aguinaga Bósquez JP, Bodor Z, Zaukuu J-LZ, Kocsis T, Zsom-Muha V, Gillay Z, Kovacs Z. Detection of Monilia Contamination in Plum and Plum Juice with NIR Spectroscopy and Electronic Tongue. Chemosensors. 2021; 9(12):355. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors9120355
Chicago/Turabian StyleVitalis, Flora, David Tjandra Nugraha, Balkis Aouadi, Juan Pablo Aguinaga Bósquez, Zsanett Bodor, John-Lewis Zinia Zaukuu, Tamás Kocsis, Viktória Zsom-Muha, Zoltan Gillay, and Zoltan Kovacs. 2021. "Detection of Monilia Contamination in Plum and Plum Juice with NIR Spectroscopy and Electronic Tongue" Chemosensors 9, no. 12: 355. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors9120355