Thermal and Structural Properties of High Density Polyethylene/Carbon Nanotube Nanocomposites: A Comparison Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodology
2.2.1. Oxidation and Amide Functionalization of CNTs
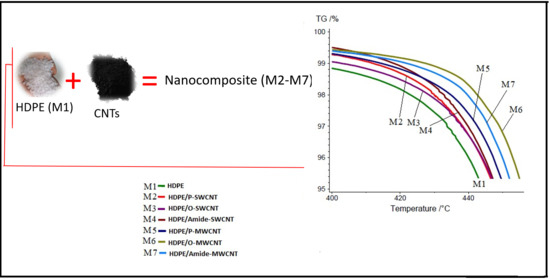
2.2.2. Preparation of HDPE/CNTs Nanocomposite Sheets
2.3. Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gabriel, L.H. History and Physical Chemistry of HDPE, Plastic Pipe Institute. 2011. Available online: https://www.yumpu.com/en/document/view/10106275/history-and-physical-chemistry-of-hdpe-plastics-pipe-institute (accessed on 3 March 2018).
- Akay, M. Introduction to Polymer Science and Technology; Ventus Publishing Aps.: Eire, Ireland, 2012; ISBN 978-87-403-0087-1. [Google Scholar]
- Simpson, D.M.; Harrison, I.R. A Study of the Effects of Processing Parameters on the Morphologies and Tensile Modulus of HDPE Blown Films: Application of Composite Theories on a Molecular Level to Characterize Tensile Modulus. J. Plast. Film Sheeting 1994, 10, 302–324. [Google Scholar] [CrossRef]
- High Density Polyethylene (HDPE) Mechanical Properties at 23 °C. Available online: https://polymerdatabase.com/Commercial%20Polymers/HDPE.html (accessed on 10 January 2021).
- Jose, J.P.; Joseph, K. Advances in Polymer Composites: Macro- and Microcomposites—State of the Art, New Challenges, and Opportunities. In Polymer Composites, 1st ed.; Thomas, S., Kuruvilla, J., Malhotra, S.K., Coda, K., Sreekala, M.S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; pp. 1–16, ISBN online 9783527645213, print 9783527326242. [Google Scholar]
- Sahu, A.K.; Sudhakar, K. Effect of UV Exposure on Bimodal HDPE Floats for Floating Solar Application. J. Mater. Res. Technol. 2019, 8, 147–156. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, J.; Xu, X. Studies on High Density Polyethylene (HDPE) Functionalized by Ultraviolet Irradiation and its Application. Polym. Int. 2003, 52, 1527–1530. [Google Scholar] [CrossRef]
- Zhang, X.; Sreekumar, T.V.; Liu, T.; Kumar, S. Properties and Structure of Nitric Acid Oxidized Single Wall Carbon Nanotube Films. J. Phys. Chem. 2004, 108, 16435–16440. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of Carbon Nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef]
- Mccrossan, K.; Mcclory, C.; Mayoral, B.; Thompson, D.; Mcconnell, D.; Mcnally, T.; Murphy, M.; Nicholson, T.; Martin, D.; Halley, P. Composites of Poly(ethyleneterephthalate) Carbon Nanotubes. In Polymer-Carbon Nanotube Composites Preparation, Properties and Application, 1st ed.; McNally, T., Potschke, P., Eds.; Woodhead Publishing Limited: Oxford, UK; Cambridge, UK; Philadelphia, PA, USA; New Delhi, India, 2011; pp. 545–586. [Google Scholar]
- Arash, B.; Wang, Q.; Varadan, V.K. Mechanical Properties of Carbon Nanotubes/Polymer Composites. Sci. Rep. 2014, 4, 6479. [Google Scholar] [CrossRef] [PubMed]
- Ruoff, R.S.; Qian, D.; Liu, W.K. Mechanical Properties of Carbon Nanotubes: Theoretical Predictions and Experimental Measurements. CR Phys. 2003, 4, 993–1008. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, H.; Qing, Q.; Yang, Y.; Li, Q.; Liu, Z.; Guo, X.; Du, Z. Effect of Chemical Oxidation on the Structure of Single-Walled Carbon Nanotubes. J. Phys. Chem. 2003, 107, 3712–3718. [Google Scholar] [CrossRef]
- Maciejewska, B.M.; Jasiurkowska-Delaporte, M.; Vasylenko, A.I.; Koziot, K.K.; Jurga, S. Experimental and Theoretical Studies on the Mechanism for Chemical Oxidation of Multiwalled Carbon Nanotubes. RSC Adv. 2014, 4, 28826–28831. [Google Scholar] [CrossRef]
- Rosca, I.D.; Watari, F.; Uo, M.; Akasaka, T. Oxidation of Multiwalled Carbon Nanotubes by Nitric acid. Carbon 2005, 43, 3124–3131. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical Oxidation of Multiwalled Carbon Nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Li, Q.W.; Yan, H.; Ye, Y.C.; Zhang, J.; Liu, Z.F. Defect Location of Individual Single-Walled Carbon Nanotubes with a Thermal Oxidation Strategy. J. Phys. Chem. Part B 2002, 106, 11085–11088. [Google Scholar]
- Osswald, S.; Havel, M.; Gogotsi, Y. Monitoring Oxidation of Multiwalled Carbon Nanotubes by Raman Spectroscopy. J. Raman Spectrosc. 2007, 38, 728–736. [Google Scholar] [CrossRef]
- Tsang, S.C.; Harris, P.J.F.; Green, M.L.H. Thinning and Opening of Carbon Nanotubes by Oxidation Using Carbon Dioxide. Nature 1993, 362, 520–522. [Google Scholar] [CrossRef]
- Yang, D.-Q.; Sacher, E. Strongly Enhanced Interaction Between Evaporated Pt Nanoparticles and Functionalized Multiwalled Carbon Nanotubes Via Plasma Surface Modifications: Effects of Physical and Chemical Defects. J. Phys. Chem. Part C 2008, 112, 4075–4082. [Google Scholar] [CrossRef]
- Zschoerper, N.P.; Katzenmaier, V.; Vohrer, U.; Haupt, M.; Oehr, C.; Hirth, T. Analytical Investigation of the Composition of Plasma-Induced Functional Groups on Carbon Nanotube Sheets. Carbon 2009, 47, 2174–2185. [Google Scholar] [CrossRef]
- Schonherr, J.; Buchheim, J.; Scholz, P.; Stelter, M. Oxidation of carbon nanotubes with ozone and hydroxyl radicals. Carbon 2017, 111, 631–640. [Google Scholar] [CrossRef]
- Koh, A.L.; Gidcumb, E.; Zhou, O.; Sinclair, R. Oxidation of Carbon Nanotubes in an Ionizing Environment. Nano Lett. 2016, 16, 856–863. [Google Scholar] [CrossRef]
- Gromov, A.; Dittmer, S.; Svensson, J.; Nerushev, O.A.; Perez-Garcia, S.A.; Licea-Jimenez, L.; Rychwalski, R.; Campbell, E.E.B. Covalent Amino-functionalisation of Single-Wall Carbon Nanotubes. J. Mater. Chem. 2005, 15, 3334–3339. [Google Scholar] [CrossRef]
- Li, M.; Boggs, M.; Beebe, T.P.; Huang, C.P. Oxidation of Single-Walled Carbon Nanotubes in Dilute Aqueous Solutions by Ozone as Affected by Ultrasound. Carbon 2008, 46, 466–475. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Popova, I.; Yates, J.T., Jr.; Bronikowski, M.J.; Huffman, C.B.; Liu, J.; Smalley, R.E.; Hwu, H.H.; Chen, J.G. Oxygen-Containing Functional Groups on Single-Wall Carbon Nanotubes: NEXAFS and Vibrational Spectroscopic Studies. J. Am. Chem. Soc. 2001, 123, 10699. [Google Scholar] [CrossRef]
- Chiang, Y.; Lin, W.; Chang, Y.C. The influence of Treatment Duration on Multi-Walled Carbon Nanotubes Functionalized by H2SO4/HNO3 Oxidation. Appl. Surf. Sci. 2011, 257, 2401. [Google Scholar] [CrossRef]
- Rahmat, M.; Hubert, P. Carbon Nanotube-Polymer Interactions in Nanocomposites: A Review. Compos. Sci. Technol. 2011, 72, 72–84. [Google Scholar] [CrossRef]
- Yang, Y.K.; China, P.R.; XIE, X.L.; China, Y.W.; Mai, Y.W. Functionalization of Carbon Nanotubes for Polymer Nanocomposites. In Polymer-Carbon Nanotube Composites Preparation, Properties and Application, 1st ed.; McNally, T., Potschke, P., Eds.; Woodhead Publishing Limited: Oxford, UK; Cambridge, UK; Philadelphia, PA, USA; New Delhi, India, 2011; pp. 55–84. [Google Scholar]
- Chen, L.; Xie, H.; Yu, W. Functionalization Methods of Carbon Nanotubes and its Applications. In Carbon Nanotubes Applications on Electron Devices, 1st ed.; Marulanda, J., Ed.; Tech Europe, University Campus sTeP Ri: Rijeks, Coroatia; Tech China: Shanghai, China, 2011; pp. 213–232. [Google Scholar]
- Camargo, P.H.C.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: Synthesis, Structure, Properties and New Application Opportunities. Mater. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef] [Green Version]
- Moniruzzaman, M.; Winey, K.I. Polymer Nanocomposites Containing Carbon Nanotubes. Macromolecules 2006, 39, 5194–5205. [Google Scholar] [CrossRef]
- Kasaliwal, G.R.; Villmow, S.P.; Potschke, P. Influence of Material and Processing Parameters on Carbon Nanotube Dispersion in Polymer Melts. In Polymer-Carbon Nanotube Composites Preparation, Properties and Application, 1st ed.; McNally, T., Potschke, P., Eds.; Woodhead Publishing Limited: Oxford, UK; Cambridge, UK; Philadelphia, PA, USA; New Delhi, India, 2011; pp. 99–132. [Google Scholar]
- Tang, W.; Santare, M.H.; Advani, S.G. Melt Processing and Mechanical Property Characterization of Multi-Walled Carbon Nanotube/High Density polyethylene (MWNT/HDPE) Composite Films. Carbon 2003, 41, 2279–2785. [Google Scholar] [CrossRef]
- Arora, G.; Pathak, H.; Zafar, S. Fabrication and Characterization of Microwave Cured High-Density Polyethylene/Carbon Nanotube and Polypropylene/Carbon Nanotube Composites. J. Compos. Mater. 2019, 53, 2091–2104. [Google Scholar] [CrossRef]
- Gao, J.; Shen, Y.; Li, C. Fabrication of High-Density Polyethylene/Multiwalled Carbon Nanotube Composites Via Submerged Friction Stir Processing: Evaluation of Morphological, Mechanical, and Thermal Behavior. J. Thermoplast. Compos. Mater. 2017, 30, 241–254. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Menezes, B.R.C.; Franceschi, W.; Ferreira, E.V.; Lozano, K.; Cividanes, L.S.; Coutinho, A.R.; Thim, G.P. Influence of Carbon Nanotube Concentration and Sonication Temperature on Mechanical Properties of HDPE/CNT Nanocomposites. Fuller. Nanotub. Carbon Nanostruct. 2017, 25, 531–539. [Google Scholar] [CrossRef]
- Al-Harthi, M.A.; Bahuleyan, B.K. Mechanical Properties of Polyethylene-Carbon Nanotube Composites Synthesized by In Situ Polymerization Using Metallocene Catalysts. Adv. Mater. Sci. Eng. 2018, 2018, 4057282. [Google Scholar] [CrossRef] [Green Version]
- Park, C.; Ounaies, Z.; Watson, K.A.; Crooks, R.E.; Smith, J.J.; Lowther, S.E.; Connell, J.W.; Siochi, E.J.; Harrison, J.S.; Clair, T.L.S. Dispersion of Single Wall Carbon Nanotubes by in Situ Polymerization Under Sonication. Chem. Phys. Lett. 2002, 364, 303–308. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon Nanotube-Polymer Composites: Chemisty, Processing, Mechanical and Electrical Properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Rana, S.; Cho, J.W.; Li, L.; Chan, S.H. Polymer Nanocomposites Based on Functionalized Carbon Nanotubes. Prog. Polym. Sci. 2010, 35, 837–867. [Google Scholar] [CrossRef]
- Jagtap, S.B.; Ratna, D. Preparation and Characterization of Rubbery Epoxy/Multiwlled Carbon Nanotubes Composites Using Amino Acid Salt Assisted Dispersion Technique. Express Polym. Lett. 2013, 7, 329–339. [Google Scholar] [CrossRef]
- Czigany, T.; Deak, T. Preparation and Manufacturing Techniqes for Macro- and Microcomposites. In Polymer Composites; Thomas, S., Kuruvilla, J., Malhotra, S.K., Goda, K., Sreekala, M.S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; Volume 1, pp. 111–134. [Google Scholar]
- Kanagaraj, S.; Varanda, F.R.; Zhil’tsova, T.V.; Oliveira, M.S.A.; Simoes, J.A.O. Mechanical Properties of High Density Polyethylene/Carbon Nanotube Composites. Compos. Sci. Technol. 2007, 67, 3071–3077. [Google Scholar] [CrossRef]
- Ferreira, F.V.; Francisco, W.; Menezes, B.R.C.; Brito, F.S.; Coutinho, A.S.; Cividanes, L.S.; Coutinho, A.R.; Thim, G.P. Correlation of Surface Treatment, Dispersion and Mechanical Properties of HDPE/CNT Nanocomposites. Appl. Surf. Sci. 2016, 389, 921–929. [Google Scholar] [CrossRef]
- Salehi, S.; Maghmoomi, F.; Sahebian, S.; Zebarjad, S.M.; Lazzeri, A. A Study on the Effect of Carbon Nanotube Surface Modification on Mechanical and Thermal Properties of CNT/HDPE Nanocomposite. J. Thermoplast. 2019, 34, 203–220. [Google Scholar] [CrossRef]
- Nobile, M.R.; Somma, E.; Valentino, O.; Simon, G.; Neitzert, H.C. Influence of the Nanotube Oxidation on the Rheological and Electrical Properties of CNT/HDPE Composites. AIP Conf. Proc. 2016, 1736, 020150. [Google Scholar] [CrossRef]
- Zhang, Q.; Rastogi, S.; Chen, D.; Lippits, D. Low Percolation Threshold in Single-Walled Carbon Nanotube/High Density Polyethylene Composites Prepared by Melt Processing Technique. Carbon 2006, 44, 778–785. [Google Scholar] [CrossRef]
- Du, J.; Zhao, L.; Zeng, Y.; Zhang, L.; Li, F.; Liu, P.; Liu, C. Comparison of Electrical Properties between Multi-Walled Carbon Nanotube and Graphene Nanosheet/High Density Polyethylene Composites with a Segregated Network Structure. Carbon 2011, 49, 1094–1100. [Google Scholar] [CrossRef]
- Mohsin, M.E.A.; Arsad, A.; Fouad, H.; Jawaid, M.; Alothman, O.Y. Enhanced Mechanical and Thermal Properties of CNT/HDPE Nanocomposite Using MMT as Secondary Filler, Times of Polymers (TOP) and Composites. AIP Conf. Proc. 2014, 1599, 206–209. [Google Scholar] [CrossRef]
- Azizian, J.; Tahermansouri, H.; Biazar, E.; Heiari, S.; Khoei, D.C. Functionalization of Carboxylated Multiwall Nanotubes with Imidazole Derivatives and Their Toxicity Investigations. Int. J. Nanomed. 2010, 5, 907–914. [Google Scholar]
- Wepasnick, K.A.; Smith, B.A.; Bitter, J.L.; Fairbrother, D.H. Chemical and Structural Characterization of Carbon Nanotube Surfaces. Anal. Bioanal. Chem. 2010, 363, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Gillbert, M. Brydson’sPlastics Materials, 8th ed.; Elsevier (Butterworth-Heinemann): Oxford, UK; Cambridge, MA, USA, 2017. [Google Scholar]
- Kodjie, S.L.; Li, L.; Li, B.; Cai, W.; Li, C.Y.; Keating, M. Morphology and Crystallization Behavior of HDPE/CNT Nanocomposite. J. Macromol. Sci. B Phys. 2006, 45, 231–245. [Google Scholar] [CrossRef]
- Shi, X.; Jiang, B.; Wang, J.; Yang, Y. Influence of Wall Number and Surface Functionalization of Carbon Nanotubes on their Antioxidant behavior in highdensity Polyethylene. Carbon 2012, 50, 1005–1013. [Google Scholar] [CrossRef]
- Mohlala, M.S.; Ray, S.S. Preparation and Characterization of Polymer/Multi-Walled Carbon Nanotube Nanocomposites. Solid State Phenom. 2008, 140, 97–102. [Google Scholar] [CrossRef]
- Guo, C.; Sun, F.; Ling, R.; Yao, J.; Zhang, Z.; Zhang, G. Crystallization and Stress Relaxation Behaviors of UHMWPE/CNT Fibers. J. Vinyl. Addit. Technol. 2016, 24, 229–232. [Google Scholar] [CrossRef]
- Li, C.Y.; Li, L.; Cai, W.; Kodjie, S.L.; Tenneti, K.K. Nanohybrid Shish Kebabs: Periodically Functionalized Carbon Nanotubes. J. Adv. Mater. 2005, 17, 1198–1202. [Google Scholar] [CrossRef]
- McNally, T.; Potschk, P.; Halley, P.; Murphy, M.; Martin, D.; Bell, S.E.J.; Brennan, G.P.; Bein, D.; Lemoine, P.; Quinn, J.P. Polyethylene Multiwalled Carbon Nanotube Composites. Polymer 2005, 46, 8222–8232. [Google Scholar] [CrossRef]
- Yang, B.X.; Pramoda, K.P.; Xu, G.Q.; Goh, S.H. Mechanical Reinforcement of Polyethylene Using Polyethylene-Grafted Multiwalled Carbon Nanotubes. Adv. Funct. Mater. 2007, 17, 2062–2069. [Google Scholar] [CrossRef]
- Hulsey, S.; Absar, S.; Sultan, Q.N.; Sabet, S.M.; Mahfuz, H.; Khan, M. Synthesis and Characterization of UHMWPE Nanocomposite Fibers Containing Carbon Nanotubes Coated with a PVP Surfactant Layer. Polym. Compos. 2018, 39, E1025–E1033. [Google Scholar] [CrossRef]
- Trujilo, M.; Arnal, M.; Muller, A.; Laredo, E.; Bredeau, D.; Dubis, P. Thermal and Morphological Characterization of Nanocomposites Prepared by in situ Polymerization of High-Density Polyethylene on Carbon Nanotubes. Macromolecules 2007, 40, 6268–6276. [Google Scholar] [CrossRef]
- Chouit, F.; Guellati, O.; Boukhezar, S.; Harat, A.; Guerioune, M.; Badi, N. Synthesis and Characterization of HDPE/N-MWCNT Nanocomposite Films. Nanoscale Res. Lett. 2014, 9, 288–293. [Google Scholar] [CrossRef] [Green Version]
- Speakman, S.A. Introduction to X-ray Powder Diffraction Data Analysis. Material Research Science And Engineering Center. Available online: https://www.researchgate.net/file.PostFileLoader.html?id=58d22df3dc332d06c7245969&assetKey=AS%3A474618730422273%401490169331513 (accessed on 10 January 2021).
- TA123. Determination of Polymer Crystallinity by DSC . TA Instruments, 109 Lukens Drive, New Castle DE 19720, USA. Available online: http://www.tainstruments.com/pdf/literature/TA123new.pdf (accessed on 29 May 2021).
- Babaei, A.; Ghaffarian, S.R.; Khorasani, M.M.; Abdolrasouli, M.H. Thermal and Mechanical Properties of Ultra High Molecular Weight Polyethylene Fiber Reinforced High-Density Polyethylene Homocomposites: Effect of Processing Condition and Nanoclay Addition. J. Macromol. Sci. B 2014, 53, 829–847. [Google Scholar] [CrossRef]
- Kanai, Y.; Khalap, V.R.; Collins, P.G.; Grossman, J.C. Atomistic Oxidation Mechanism of a Carbon Nanotube in Nitric Acid. Phys. Rev. 2010, 104, 066401. [Google Scholar] [CrossRef] [PubMed]
- Unge, M.; Christen, T.; Tornkuist, C. Electronic structure of Polyethylene crystalline and Amorphous Phases of Pure Polyethylene and their Interfaces. In Proceedings of the 2012 Annual Report Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Montreal, QC, Canada, 14–17 October 2012. [Google Scholar] [CrossRef]
Short Biography of Authors








| Sample Name | CNTs Type | HDPE/CNTs Nanocomposite Sheet |
|---|---|---|
| M1 | Reference sample | HDPE |
| M2 | Pristine-SWCNT | HDPE/P-SWCNT |
| M3 | Oxidized-SWCNT | HDPE/O-SWCNT |
| M4 | Amide-SWCNT | HDPE/Amide-SWCNT |
| M5 | Pristine-MWCNT | HDPE/P-MWCNT |
| M6 | Oxidized-MWCNT | HDPE/O-MWCNT |
| M7 | Amide-MWCNT | HDPE/Amide-MWCNT |
| Sample Name | T95 (°C) | Ton (°C) First Derivative T | Residue Content (%) |
|---|---|---|---|
| M1 | 444 | 459.9 | 1.61 |
| M2 | 448 | 463.6 | 1.77 |
| M3 | 448 | 465.2 | 1.29 |
| M4 | 448 | 462.7 | 1.55 |
| M5 | 451 | 464.8 | 1.13 |
| M6 | 456 | 466.8 | 1.21 |
| M7 | 453 | 466.5 | 1.21 |
| Sample Name | Tm (°C) | Tc (°C) | ∆Hm (J/g) | %Crystallinity * |
|---|---|---|---|---|
| M1 | 124.8 | 109.6 | 90 | 31 |
| M2 | 125.4 | 115.3 | 87 | 30 |
| M3 | 126.1 | 115.1 | 86 | 30 |
| M4 | 125.7 | 115.4 | 82 | 28 |
| M5 | 125.0 | 114.4 | 84 | 28 |
| M6 | 124.3 | 115.1 | 89 | 31 |
| M7 | 125.3 | 114.4 | 83 | 29 |
| Sample | % Crystallinity | Grain Size (Å) | Lattice Constant (Å) |
|---|---|---|---|
| M1 | 63.4 | 155.5 | a = 7.533, b = 5.000, c = 2.542 |
| M2 | 67.2 | 139.0 | a = 7.431, b = 4.934, c = 2.477 |
| M3 | 68.0 | 152.4 | a = 7.517, b = 4.988, c = 2.493 |
| M4 | 65.4 | 164.9 | a = 7.552, b = 5.018, c = 2.496 |
| M5 | 60.5 | 152.0 | a = 7.533, b = 5.003, c = 2.548 |
| M6 | 75.3 | 128.0 | a = 7.355, b = 4.867, c = 2.529 |
| M7 | 63.8 | 140.7 | a = 7.422, b = 4.930, c = 2.527 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozeya, A.; Makableh, Y.F.; Abu-Zurayk, R.; Khalaf, A.; Al Bawab, A. Thermal and Structural Properties of High Density Polyethylene/Carbon Nanotube Nanocomposites: A Comparison Study. Chemosensors 2021, 9, 136. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors9060136
Bozeya A, Makableh YF, Abu-Zurayk R, Khalaf A, Al Bawab A. Thermal and Structural Properties of High Density Polyethylene/Carbon Nanotube Nanocomposites: A Comparison Study. Chemosensors. 2021; 9(6):136. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors9060136
Chicago/Turabian StyleBozeya, Ayat, Yahia F. Makableh, Rund Abu-Zurayk, Aya Khalaf, and Abeer Al Bawab. 2021. "Thermal and Structural Properties of High Density Polyethylene/Carbon Nanotube Nanocomposites: A Comparison Study" Chemosensors 9, no. 6: 136. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors9060136