Development of a High-Throughput Low-Cost Approach for Fabricating Fully Drawn Paper-Based Analytical Devices Using Commercial Writing Tools
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instrumentation and Signal Evaluation
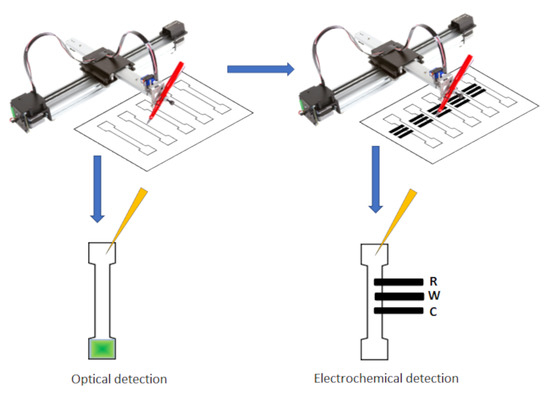
2.3. Fabrication of PADs
2.4. Optical and Electrochemical Detection
3. Results
3.1. Investigation of Type of Paper
3.2. Investigation of the Type of Marker Pen
3.3. Investigation of the PAD Fabrication Parameters
3.4. Applications in Optical and Electrochemical Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nery, E.W.; Kubota, L.T. Sensing approaches on paper-based devices: A review. Anal. Bioanal. Chem. 2013, 405, 7573–7595. [Google Scholar] [CrossRef]
- Yamada, K.; Shibata, H.; Suzuki, K.; Citterio, D. Toward practical application of paper-based microfluidics for medical diagnostics: State-of-the-art and challenges. Lab. Chip 2017, 17, 1206–1249. [Google Scholar] [CrossRef]
- Kemal Yetisen, A.; Safwan Akram, M.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic devices. Lab. Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef]
- López-Marzo, A.M.; Merkoçi, A. Paper-based sensors and assays: A success of the engineering design and the convergence of knowledge areas. Lab. Chip 2016, 16, 3150–3176. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low volume, portable bioassays. Angew. Chem. Int. Ed. Engl. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, J.C.; DeGregory, P.R.; Crooks, R.M. New Functionalities for Paper-Based Sensors Lead to Simplified User Operation, Lower Limits of Detection, and New Applications. Annu. Rev. Anal. Chem. 2016, 9, 183–202. [Google Scholar] [CrossRef] [Green Version]
- Boobphahom, S.; Nguyet Ly, M.; Soum, V.; Pyun, N.; Kwon, O.S.; Rodthongkum, N.; Shin, K. Recent Advances in Microfluidic Paper-Based Analytical Devices toward High-Throughput Screening. Molecules 2020, 25, 2970. [Google Scholar] [CrossRef] [PubMed]
- Tribhuwan Singh, A.; Lantigua, D.; Meka, A.; Taing, S.; Pandher, M.; Camci-Unal, G. Paper-Based Sensors: Emerging Themes and Applications. Sensors 2018, 18, 2838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suntornsuk, W.; Suntornsuk, L. Recent applications of paper-based point-of-care devices for biomarker detection. Electrophoresis 2020, 41, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.T.; Hou, C.Y.; Wang, Y.N.; Fu, L.M. Microfluidic paper-based analytical devices for environmental analysis of soil, air, ecology and river water. Sens. Actuators B Chem. 2019, 301, 126855. [Google Scholar] [CrossRef]
- Fu, L.M.; Wang, Y.N. Detection methods and applications of microfluidic paper-based analytical devices. Trends Anal. Chem. 2018, 107, 196–211. [Google Scholar] [CrossRef]
- Lim, H.; Turab Jafry, A.; Lee, J. Fabrication, Flow Control, and Applications of Microfluidic Paper-Based Analytical Devices. Molecules 2019, 24, 2869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozer, T.; McMahon, C.; Henry, C.S. Advances in Paper-Based Analytical Devices. Annu. Rev. Anal. Chem. 2020, 13, 85–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akyazi, T.; Basabe-Desmonts, L.; Benito-Lopez, F. Review on microfluidic paper-based analytical devices towards commercialization. Anal. Chim. Acta 2018, 1001, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, H.; He, X.; Xu, F.; Li, F. Pen-on-paper strategies for point-of-care testing of human health. Trends Anal. Chem. 2018, 108, 50–64. [Google Scholar] [CrossRef]
- Nishat, S.; Jafry, A.T.; Martinez, A.W.; Awan, F.R. Paper-based microfluidics: Simplified fabrication and assay methods. Sens. Actuators B Chem. 2021, 336, 129681. [Google Scholar] [CrossRef]
- Jiang, X.; Hugh Fan, Z. Fabrication and Operation of Paper-Based Analytical Devices. Annu. Rev. Anal. Chem. 2016, 9, 203–222. [Google Scholar] [CrossRef]
- Ramesh Singhal, H.; Prabhu, A.; Nandagopal, M.S.G.; Dheivasigamani, D.; Kumar Mani, N. One-dollar microfluidic paper-based analytical devices: Do-It-Yourself approaches. Microchem. J. 2021, 165, 106126. [Google Scholar] [CrossRef]
- Lu, Y.; Shi, W.; Jiang, L.; Qin, J.; Lin, B. Rapid prototyping of paper-based microfluidics with wax for low-cost, portable bioassay. Electrophoresis 2009, 30, 1497–1500. [Google Scholar] [CrossRef]
- Yang, H.; Kong, Q.; Wang, S.; Xu, J.; Bian, Z.; Zheng, X.; Ma, C.; Ge, S.; Yu, J. Hand-drawn & written pen-on-paper electrochemiluminescence immunodevice powered by rechargeable battery for low-cost point-of-care testing. Biosens. Bioelectron. 2014, 61, 21–27. [Google Scholar]
- Abedi Ostad, M.; Hajinia, A.; Heidari, T. A novel direct and cost effective method for fabricating paper-based microfluidic device by commercial eye pencil and its application for determining simultaneous calcium and magnesium. Microchem. J. 2017, 133, 545–550. [Google Scholar] [CrossRef]
- Li, Z.; Li, F.; Xing, Y.; Liu, Z.; You, M.; Li, Y.; Wen, T.; Qu, Z.; Li, X.L.; Xu, F. Pen-on-paper strategy for point-of-care testing: Rapid prototyping of fully written microfluidic biosensor. Biosens. Bioelectron. 2017, 98, 478–485. [Google Scholar] [CrossRef]
- Gomes Aguilar, L.; Marques Petroni, J.; Souza Ferreira, V.; Gabriel Lucca, B. Easy and rapid pen-on-paper protocol for fabrication of paper analytical devices using inexpensive acrylate-based plastic welding repair kit. Talanta 2020, 219, 121246. [Google Scholar] [CrossRef]
- Kumar Mani, N.; Prabhu, A.; Kumar Biswas, S.; Chakraborty, S. Fabricating Paper Based Devices Using Correction Pens. Sci. Rep. 2019, 9, 1752. [Google Scholar] [CrossRef]
- Varsha, V.; Aishwarya, S.; Murchana, S.; Naveen, G.; Ramya, M.; Rathinasabapathi, P. Correction pen based paper fluidic device for the detection of multiple gene targets of Leptospira using Loop Mediated Isothermal Amplification. J. Microbiol. Methods 2020, 174, 105962. [Google Scholar] [CrossRef]
- Sousa, L.R.; Duarte, L.C.; Coltro, W.K.T. Instrument-free fabrication of microfluidic paper-based analytical devices through 3D pen drawing. Sens. Actuators B Chem. 2020, 312, 128018. [Google Scholar] [CrossRef]
- Oyola-Reynoso, S.; Heim, A.P.; Halbertsma-Black, J.; Zhao, C.; Tevis, I.D.; Çınar, S.; Cademartiri, R.; Liu, X.; Bloch, J.F.; Thuo, M.M. Draw your assay: Fabrication of low-cost paper-based diagnostic and multi-well test zones by drawing on a paper. Talanta 2015, 145, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Satarpai, T.; Siripinyanond, A. Alternative Patterning Methods for Paper-based Analytical Devices Using Nail Polish as a Hydrophobic Reagen. Anal. Sci. 2018, 34, 605–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamidon, N.N.; Hong, Y.; Salentijn, G.I.J.; Verpoorte, E. Water-based alkyl ketene dimer ink for user-friendly patterning in paper microfluidics. Anal. Chim. Acta 2018, 1000, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Guo, P.; Guan, P.; Wang, S.; Lei, Y.; Wang, G. The fabrication of paper separation channel based SERS substrate and its recyclable separation and detection of pesticides. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 240, 118561. [Google Scholar] [CrossRef]
- Nuchtavorn, N.; Macka, M. A novel highly flexible, simple, rapid and low-cost fabrication tool for paper-based microfluidic devices (mPADs) using technical drawing pens and in-house formulated aqueous inks. Anal. Chim. Acta 2016, 919, 70–77. [Google Scholar] [CrossRef]
- Dhavamani, J.; Hamid Mujawar, L.; Soror El-Shahawi, M. Hand drawn paper-based optical assay plate for rapid and trace level determination of Ag+ in water. Sens. Actuators B Chem. 2018, 258, 321–330. [Google Scholar] [CrossRef]
- Nie, J.; Zhang, Y.; Lin, L.; Zhou, C.; Li, S.; Zhang, L.; Li, J. Low-Cost Fabrication of Paper-Based Microfluidic Devices by One-Step Plotting. Anal. Chem. 2012, 84, 6331–6335. [Google Scholar] [CrossRef] [PubMed]
- Dossi, N.; Petrazzi, S.; Toniolo, R.; Tubaro, F.; Terzi, F.; Piccin, E.; Svigelj, R.; Bontempelli, G. Digitally Controlled Procedure for Assembling Fully Drawn Paper-Based Electroanalytical Platforms. Anal. Chem. 2017, 89, 10454–10460. [Google Scholar] [CrossRef]
- Ghaderinezhad, F.; Amin, R.; Temirel, M.; Yenilmez, B.; Wentworth, A.; Tasoglu, S. High-throughput rapid-prototyping of low-cost paper-based microfluidics. Sci. Rep. 2017, 7, 3553. [Google Scholar] [CrossRef]
- Amin, R.; Ghaderinezhad, F.; Li, L.; Lepowsky, E.; Yenilmez, B.; Knowlton, S.; Tasoglu, S. Continuous-Ink, Multiplexed Pen-Plotter Approach for Low-Cost, High-Throughput Fabrication of Paper-Based Microfluidics. Anal. Chem. 2017, 89, 6351–6357. [Google Scholar] [CrossRef] [PubMed]
- Ghosale, A.; Shankar, R.; Ganesand, V.; Shrivas, K. Direct-Writing of Paper Based Conductive Track using Silver Nano-ink for Electroanalytical Application. Electrochim. Acta 2016, 209, 511–520. [Google Scholar] [CrossRef]
- Soum, V.; Cheong, H.; Kim, K.; Kim, Y.; Chuong, M.; Ryeon Ryu, S.; Ki Yuen, P.; Kwon, O.S.; Shin, K. Programmable Contact Printing Using Ballpoint Pens with a Digital Plotter for Patterning Electrodes on Paper. ACS Omega 2018, 3, 16866–16873. [Google Scholar] [CrossRef]
- Li, Z.; Li, F.; Hu, J.; Hong Wee, W.; Long Han, Y.; Pingguan-Murphy, B.; Jian Lu, T.; Xu, F. Direct writing electrodes using a ball pen for paper-based point-of-care testing. Analyst 2015, 140, 5526–5535. [Google Scholar] [CrossRef]
- Yukird, J.; Soum, V.; Kwon, O.S.; Shin, K.; Chailapakul, O.; Rodthongkum, N. 3D paper-based microfluidic device: A novel dual-detection platform of bisphenol A. Analyst 2020, 145, 1491–1498. [Google Scholar] [CrossRef]
- Dias, A.A.; Cardoso, T.M.G.; Chagas, C.L.S.; Oliveira, V.X.G.; Munoz, R.A.A.; Henry, C.S.; Santana, M.H.P.; Paixa, T.R.L.C.; Coltro, W.K.T. Detection of Analgesics and Sedation Drugs in Whiskey Using Electrochemical Paper-based Analytical Devices. Electroanalysis 2018, 30, 2250–2257. [Google Scholar] [CrossRef]
- Younes Jomma, E.; Bao, N.; Ding, S.N. A pencil drawn microelectrode on paper and its application in two-electrode electrochemical sensors. Anal. Methods 2017, 9, 3513–3518. [Google Scholar] [CrossRef]
- Li, W.; Qian, D.; Li, Y.; Bao, N.; Gu, H.; Yu, C. Fully-drawn pencil-on-paper sensors for electroanalysis of dopamine. J. Electroanal. Chem. 2016, 769, 72–79. [Google Scholar] [CrossRef]
- Otávio Orzaria, L.; Aparecida de Araujo Andreottia, I.; Fernando Bergamini, M.; Humberto Marcolino Junior, L.; Campos Janegitz, B. Disposable electrode obtained by pencil drawing on corrugated substrate. Sens. Actuators B Chem. 2018, 264, 20–26. [Google Scholar] [CrossRef]
- Dossi, N.; Toniolo, R.; Impellizzieri, F.; Tubaro, F.; Bontempelli, G.; Terzi, F.; Piccin, E. A paper-based platform with a pencil-drawn dual amperometric detector for the rapid quantification of ortho-diphenols in extravirgin olive oil. Anal. Chim. Acta 2017, 950, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Slinkard, K.; Singleton, V.L. Total Phenol Analysis: Automation and Comparison with Manual Methods. Am. J. Enol. Viticul. 1977, 28, 49–55. [Google Scholar]
- Kit-Anan, W.; Olarnwanich, A.; Sriprachuabwong, C.; Karuwan, C.; Tuantranont, A.; Wisitsoraat, A.; Srituravanich, W.; Pimpin, A. Disposable paper-based electrochemical sensor utilizing inkjet-printed polyaniline-modified screen-printed carbon electrode for ascorbic acid detection. J. Electroanal. Chem. 2021, 685, 72–78. [Google Scholar] [CrossRef]
- Nunez-Bajo, E.; Fernández-Abedul, M.T. Paper-based platforms with coulometric readout for ascorbic acid determination in fruit juices. Analyst 2020, 145, 3431–3439. [Google Scholar] [CrossRef]





| Paper No. | Type of Paper | Nominal Speed | Thickness | Weight | Pore Size | Longitudinal Migration |
|---|---|---|---|---|---|---|
| 1 | Whatman grade 2 filter paper | 240 s/100 mL (Herzberg) * | 190 μm | 97 g/m2 | 8 μm | G |
| 2 | Munktell grade 1288 filter paper | 70 s/100 mL (Herzberg) * | 210 μm | 84 g/m2 | 12–15 μm | P |
| 3 | Macherey-Nagel grade MN 640w filter paper | 9 s/10 mL (DIN 53137) * | 200 μm | 85 g/m2 | 8–12 μm | P |
| 4 | Whatman grade 42 filter paper | 1870 s/100 mL (Herzberg) | 200 μm | 100 g/m2 | 2.5 μm | G |
| 5 | Whatman grade 1 chromatography paper | 130 mm/30 min ** | 180 μm | 87 g/m2 | NR | G |
| 6 | Whatman grade 1 filter paper | 150 s/100 mL (Herzberg) * | 180 μm | 87 g/m2 | 11 μm | G |
| 7 | Macherey-Nagel grade MN 261 | 90–100mm/30 min ** | 180 μm | 90 g/m2 | NR | G |
| 8 | Low-cost no brand filter paper | NR * | NR | NR | NR | P |
| Pen No. | Type of Pen | Channel Isolation |
|---|---|---|
| 1 | Staedtler permanent Lumocolor waterproof 0.4 mm (s) ((a) blue, (b) black, (c) red) | ds |
| 2 | Unipin fine line waterproof PIN 08-200 0.8 mm | no |
| 3 | Edding 8055 outdoor marker waterproof 1–2 mm | no |
| 4 | Edding 791 paint marker waterproof 1–2 mm | no |
| 5 | Shachihata Artline paint marker EK-440XF 1–2 mm (xylene free) | no |
| 6 | Edding 300 permanent marker water-resistant 1.5–3 mm | ds |
| 7 | Shachihata Artline laundry marker EK-770 0.7 mm (xylene free) | no |
| 8 | Grand Paint Marker Olejowy paint marker GR-25 1.8 mm | ds |
| 9 | Stabilo point 88/11 fine 0.4 mm (light blue) | no |
| 10 | Stabilo pen 68/16 1 mm (green) | no |
| 11 | Donau paintmarker D-oil permanent 2.2 mm (Yellow) | no |
| 12 | Shachihata Artline Freezer bag marker EK-770 1 mm (xylene free) ((a) black, (b) blue) | no |
| 13 | Edding 780 0.8 mm ((a) blue, (b) black) | ds |
| 14 | United Office metallic marker 0.8 mm (F) HG02687E ((a) silver, (b) gold) | no |
| 15 | Faber Castell Multimark 1523 0.4 mm (s) ((a) black, (b) blue, (c) red) | no |
| 16 | BIC Marking Ultra Fine point permanent marker 0.6 mm (black) | no |
| 17 | BIC Marking Pro ultra-resistant permanent marker 1.1 mm | ss |
| Type of Paper | Line Thickness ± SD (mm) * | Contact Angle (°) * |
|---|---|---|
| Paper 1 | 2.8 ± 0.1 | 124 ± 4.9 |
| Paper 4 | 2.0 ± 0.2 | 117 ± 4.2 |
| Paper 5 | 2.3 ± 0.2 | 115 ± 6.4 |
| Paper 6 | 3.0 ± 0.3 | 116 ± 5.1 |
| Paper 7 | 2.2 ± 0.2 | 117 ± 4.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagkali, V.; Stavra, E.; Soulis, D.; Economou, A. Development of a High-Throughput Low-Cost Approach for Fabricating Fully Drawn Paper-Based Analytical Devices Using Commercial Writing Tools. Chemosensors 2021, 9, 178. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors9070178
Pagkali V, Stavra E, Soulis D, Economou A. Development of a High-Throughput Low-Cost Approach for Fabricating Fully Drawn Paper-Based Analytical Devices Using Commercial Writing Tools. Chemosensors. 2021; 9(7):178. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors9070178
Chicago/Turabian StylePagkali, Varvara, Eleftheria Stavra, Dionysios Soulis, and Anastasios Economou. 2021. "Development of a High-Throughput Low-Cost Approach for Fabricating Fully Drawn Paper-Based Analytical Devices Using Commercial Writing Tools" Chemosensors 9, no. 7: 178. https://0-doi-org.brum.beds.ac.uk/10.3390/chemosensors9070178