Anti-Anaphylactic Activity of Isoquercitrin (Quercetin-3-O-β-d-Glucose) in the Cardiovascular System of Animals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Substance and Reagents
2.2. Experimental Animals
2.3. Cardiovascular Anaphylaxis in Pithed Rats
2.4. Anaphylaxis in Isolated Guinea Pig Hearts
2.5. Anaphylaxis in Isolated Guinea Pig Mesenteric Arterial Beds
2.6. Anaphylaxis in Isolated Guinea Pig Pulmonary Artery
2.7. Histamine Release in Rat Peritoneal Mast Cells
2.8. Antagonism of IQ Against Histamine in Isolated Guinea Pig Guinea Trachea and Ileum
2.9. Analysis of Histamine Concentration and Creatine Kinase Activity
2.10. Intracellular Calcium Level Measurement
2.11. Statistical Analysis
3. Results
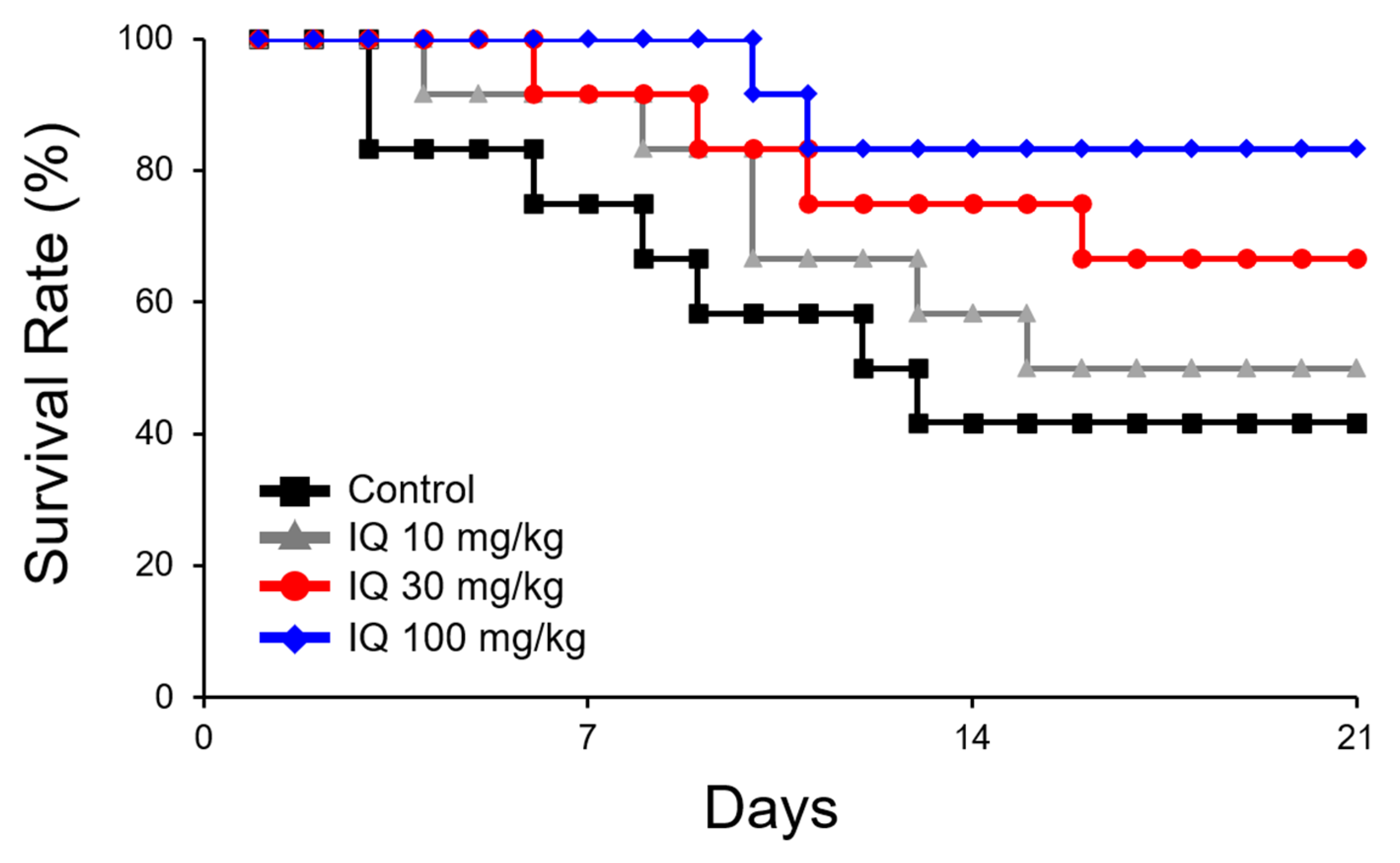
3.1. IQ Alleviates Cardiovascular Anaphylaxis in Pithed Rats
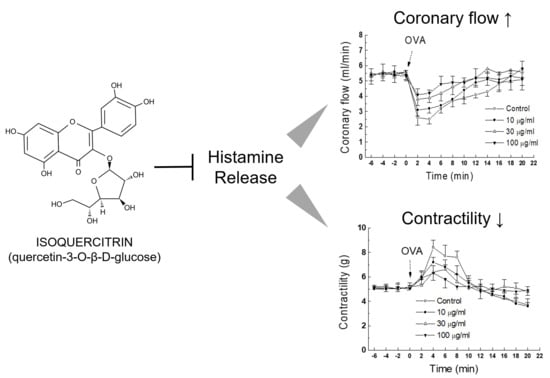
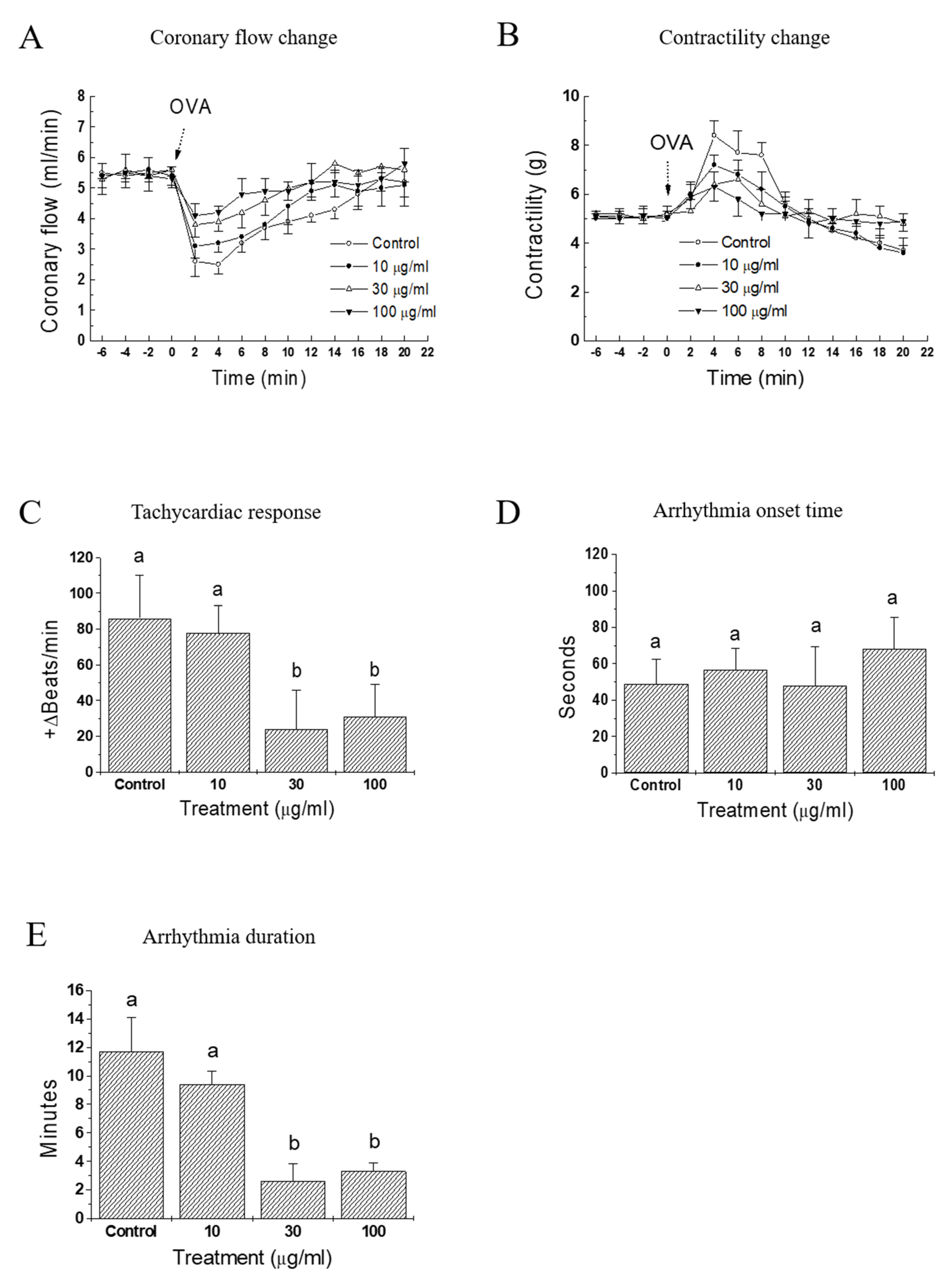
3.2. IQ Improves Cardiac Anaphylaxis in Isolated Guinea Pig Heart
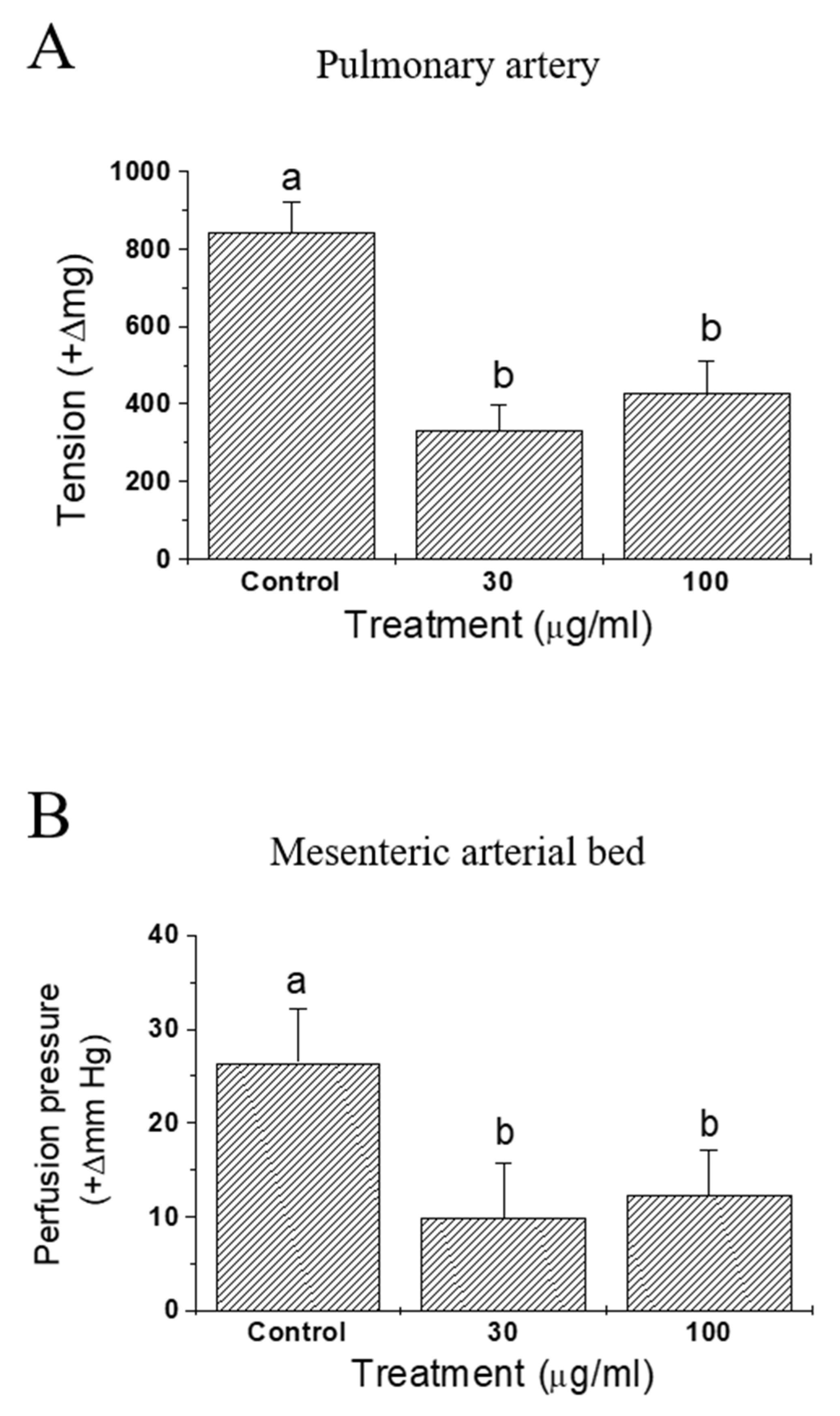
3.3. IQ Ameliorates Anaphylaxis in Isolated Vasculatures of Guinea Pig Heart
3.4. IQ Suppresses Histamine and Creatinine Release in Isolated Guinea Pig Heart and Rat Mast Cells
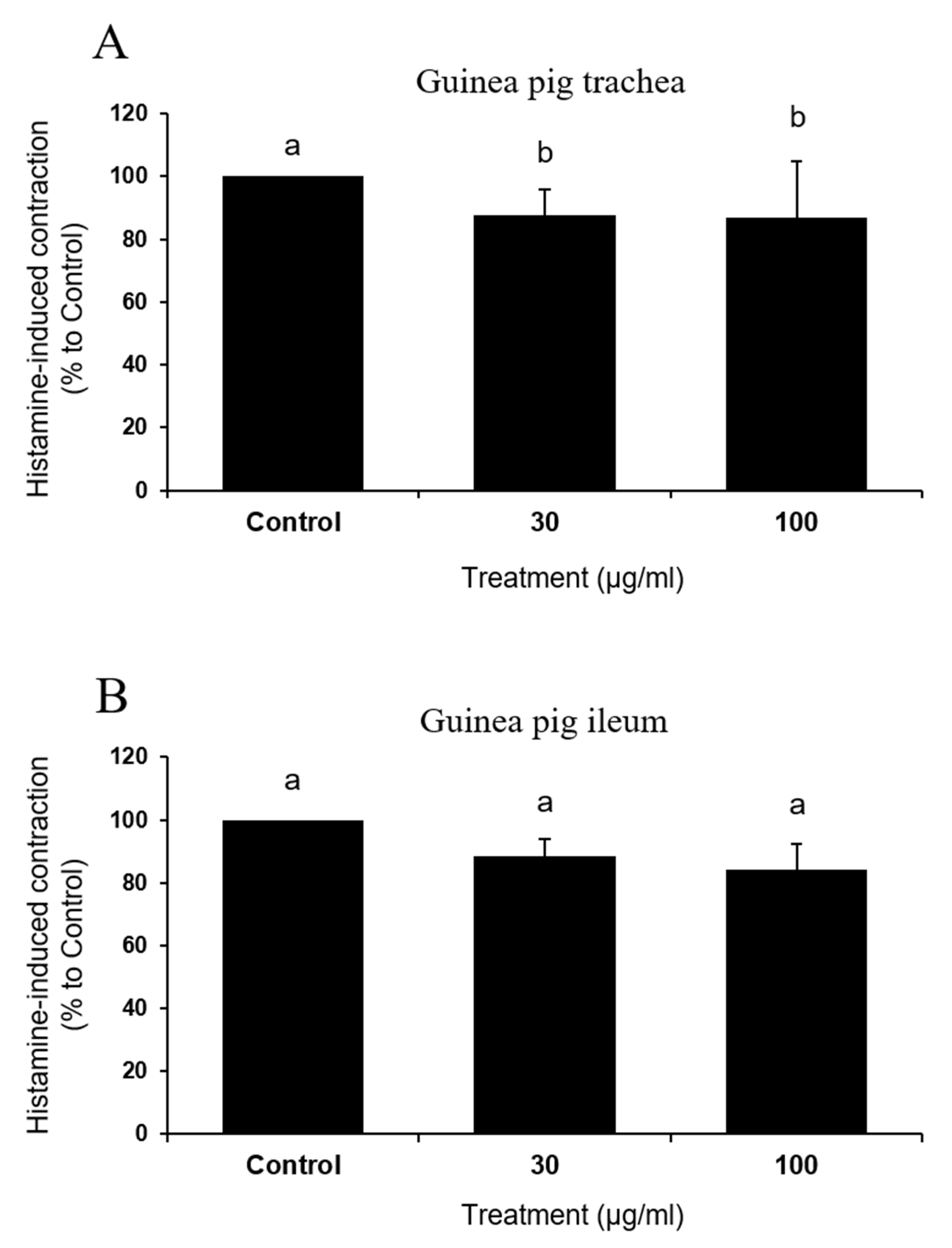
3.5. IQ Did not Affect Direct Muscle Contraction by Histamine in Tissues Isolated from Guinea Pig
3.6. IQ Stabilizes Mast Cells and Inhibits OVA-Stimulated Intracellular Calcium Release
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Lieberman, P.; Camargo, C.A., Jr.; Bohlke, K.; Jick, H.; Miller, R.L.; Sheikh, A.; Simons, F.E.R. Epidemiology of anaphylaxis: Findings of the American College of Allergy, Asthma, and Immunology Epidemiology of Anaphylaxis Working Group. Ann. Allergy Asthma Immunol. 2006, 97, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Lucke, W.C.; Thomas, H. Anaphylaxis: Pathophysiology, clinical presentations, and treatment. J. Emerg. Med. 1983, 1, 83–95. [Google Scholar] [CrossRef]
- Triggiani, M.; Patella, V.; Staiano, R.I.; Granata, F.; Marone, G. Allergy and the cardiovascular system. Clin. Exp. Immunol. 2008, 153, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Formica, J.; Regelson, W. Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Fernández, J.; Reyes, R.; Ponce, H.; Oropeza, M.; van Calsteren, M.-R.; Jankowski, C.; Campos, M.G.; Reyes-Chilpa, R. Isoquercitrin from Argemone platyceras inhibits carbachol and leukotriene D4-induced contraction in guinea-pig airways. Eur. J. Pharmacol. 2005, 522, 108–115. [Google Scholar] [CrossRef]
- Silva, C.; Raulino, R.; Cerqueira, D.; Mannarino, S.; Pereira, M.; Panek, A.; Silva, J.; Menezes, F.; Eleutherio, E.C.A. In vitro and in vivo determination of antioxidant activity and mode of action of isoquercitrin and Hyptis fasciculata. Phytomedicine 2009, 16, 761–767. [Google Scholar] [CrossRef]
- Jung, S.H.; Kim, B.J.; Lee, E.H.; Osborne, N.N. Isoquercitrin is the most effective antioxidant in the plant Thuja orientalis and able to counteract oxidative-induced damage to a transformed cell line (RGC-5 cells). Neurochem. Int. 2010, 57, 713–721. [Google Scholar] [CrossRef]
- Junior, A.G.; Junior, A.G.; Lourenço, E.L.B.; Crestani, S.; Stefanello, M.; Élida, A.; Salvador, M.; da Silva-Santos, J.E.; Marques, M.C.A.; Kassuya, C.A.L. Antihypertensive effects of isoquercitrin and extracts from Tropaeolum majus L.: Evidence for the inhibition of angiotensin converting enzyme. J. Ethnopharmacol. 2011, 134, 363–372. [Google Scholar] [CrossRef] [Green Version]
- Amado, N.G.; Cerqueira, D.M.; Menezes, F.S.; da Silva, J.F.M.; Neto, V.M.; Abreu, J.G. Isoquercitrin isolated from Hyptis fasciculata reduces glioblastoma cell proliferation and changes β-catenin cellular localization. Anti-Cancer Drugs 2009, 20, 543–552. [Google Scholar] [CrossRef]
- Junior, A.G.; Prando, T.B.L.; Leme, T.D.S.V.; Junior, A.G.; Lourenço, E.L.B.; Rattmann, Y.D.; Da Silva-Santos, J.E.; Kassuya, C.A.L.; Marques, M.C.A. Mechanisms underlying the diuretic effects of Tropaeolum majus L. extracts and its main component isoquercitrin. J. Ethnopharmacol. 2012, 141, 501–509. [Google Scholar] [CrossRef]
- Itoh, T.; Ohguchi, K.; Nakajima, C.; Oyama, M.; Iinuma, M.; Nozawa, Y.; Akao, Y.; Ito, M. Inhibitory effects of flavonoid glycosides isolated from the peel of Japanese persimmon (Diospyros kaki Fuyu) on antigen-stimulated degranulation in rat basophilic leukaemia RBL-2H3 cells. Food Chem. 2011, 126, 289–294. [Google Scholar] [CrossRef]
- Park, H.-H.; Lee, S.; Son, H.-Y.; Park, S.-B.; Kim, M.-S.; Choi, E.-J.; Singh, T.S.K.; Ha, J.-H.; Lee, M.-G.; Kim, J.-E.; et al. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch. Pharmacal Res. 2008, 31, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Koh, D.; Kim, K.; Park, J.; Lim, Y. Antiallergic activity of a disaccharide isolated fromSanguisorba of? Cinalis. Phytotherapy Res. 2004, 18, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Rubin, L.E.; Gross, S.S.; Levi, R. Nitric oxide is a mediator of hypoxic coronary vasodilatation. Relation to adenosine and cyclooxygenase-derived metabolites. Circ. Res. 1992, 71, 992–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.-S.; Park, K.-H.; Kwon, K.-B.; Mun, B.-S.; Song, C.-M.; Song, Y.-S.; Seo, E.-A.; Kim, Y.-S.; Kim, K.-J.; Ryu, -G. Anti-allergic Activity of the Sophorae Radix Water Extract in Experimental Animals. Am. J. Chin. Med. 2001, 29, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Long, J.P.; Cannon, J.G. Effects of serotonin1-like receptor agonists on autonomic neurotransmission. Can. J. Physiol. Pharmacol. 1991, 69, 1855–1860. [Google Scholar] [CrossRef] [PubMed]
- Shore, P.; Burkhalter, A.; Cohn, V.H. A method for the fluorometric assay of histamine in tissues. J. Pharmacol. Exp. Ther. 1959, 127, 182–186. [Google Scholar]
- Xu, F.; Zhuang, J.; Zhou, T.; Lee, L.-Y. Ovalbumin sensitization alters the ventilatory responses to chemical challenges in guinea pigs. J. Appl. Physiol. 2005, 99, 1782–1788. [Google Scholar] [CrossRef] [Green Version]
- del Balzo, U.; Polley, M.J.; Levi, R. Cardiac anaphylaxis. Complement activation as an amplification system. Circ. Res. 1989, 65, 847–857. [Google Scholar] [CrossRef] [Green Version]
- Feigen, G.; Prager, D.J. Experimental cardiac anaphylaxis. Physiologic, pharmacologic, and biochemical aspects of immune reactions in the isolated heart. Am. J. Cardiol. 1969, 24, 474–491. [Google Scholar] [CrossRef]
- Levi, R.; Burke, J.; Corey, E.J. SRS-A, leukotrienes, and immediate hypersensitivity reactions of the heart. Adv. Prostaglandin Thromboxane Leukot. Res. 1982, 9, 215–222. [Google Scholar] [PubMed]
- Vleeming, W.; van Rooij, H.H.; Werner, J.; Porsius, A.J. Characterization and Modulation of Antigen-Induced Effects in Isolated Rat Heart. J. Cardiovasc. Pharmacol. 1991, 18, 556–565. [Google Scholar] [CrossRef] [PubMed]
- MacGlashan, D. Histamine. J. Allergy Clin. Immunol. 2003, 112, 53–59. [Google Scholar] [CrossRef]
- Castillo, M.; Scott, N.; Mustafa, M.; Mustafa, M.S.; Azuara-Blanco, A. Topical antihistamines, and mast cell stabilisers for treating seasonal and perennial allergic conjunctivitis. Cochrane Database Syst. Rev. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.-H.; Akazawa, H.; Tamagawa, M.; Ito, K.; Yasuda, N.; Kudo, Y.; Yamamoto, R.; Ozasa, Y.; Fujimoto, M.; Wang, P.; et al. Cardiac mast cells cause atrial fibrillation through PDGF-A–mediated fibrosis in pressure-overloaded mouse hearts. J. Clin. Investig. 2010, 120, 242–253. [Google Scholar] [CrossRef] [Green Version]
- Ring, J.; Beyer, K.; Biedermann, T.; Bircher, A.; Duda, D.; Fischer, J.; Friedrichs, F.; Fuchs, T.; Gieler, U.; Jakob, T.; et al. Guideline for acute therapy and management of anaphylaxis. Allergo J. Int. 2014, 23, 96–112. [Google Scholar] [CrossRef]
- Kahraman, A.; Erkasap, N.; Köken, T.; Serteser, M.; Aktepe, F.; Erkasap, S. The antioxidative and antihistaminic properties of quercetin in ethanol-induced gastric lesions. Toxicology 2003, 183, 133–142. [Google Scholar] [CrossRef]
- Cruz, E.; Da-Silva, S.; Muzitano, M.; Silva, P.; Costa, S.S.; Rossi-Bergmann, B. Immunomodulatory pretreatment with Kalanchoe pinnata extract and its quercitrin flavonoid effectively protects mice against fatal anaphylactic shock. Int. Immunopharmacol. 2008, 8, 1616–1621. [Google Scholar] [CrossRef]
- Matsumoto, T.; Horiuchi, M.; Kamata, K.; Seyama, Y. Effects of Bidens pilosa L. var. radiata SCHERFF treated with enzyme on histamine-induced contraction of guinea pig ileum and on histamine release from mast cells. J. Smooth Muscle Res. 2009, 45, 75–86. [Google Scholar] [CrossRef]
- Kim, H.Y.; Yoon, J.H.; Yokozawa, T.; Sakata, K.; Lee, S. Protective activity of flavonoid and flavonoid glycosides against glucose-mediated protein damage. Food Chem. 2011, 126, 892–895. [Google Scholar] [CrossRef]



© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J. Anti-Anaphylactic Activity of Isoquercitrin (Quercetin-3-O-β-d-Glucose) in the Cardiovascular System of Animals. Biomedicines 2020, 8, 139. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines8060139
Park J. Anti-Anaphylactic Activity of Isoquercitrin (Quercetin-3-O-β-d-Glucose) in the Cardiovascular System of Animals. Biomedicines. 2020; 8(6):139. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines8060139
Chicago/Turabian StylePark, Jinbong. 2020. "Anti-Anaphylactic Activity of Isoquercitrin (Quercetin-3-O-β-d-Glucose) in the Cardiovascular System of Animals" Biomedicines 8, no. 6: 139. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines8060139