GPR171 Activation Modulates Nociceptor Functions, Alleviating Pathologic Pain
Abstract
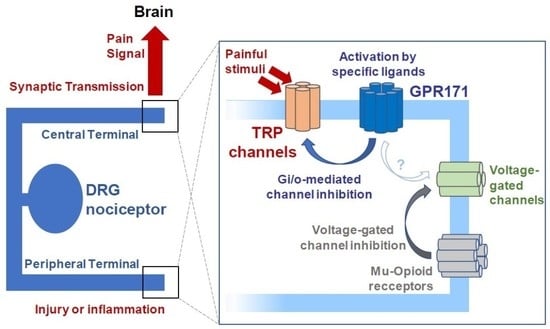
:1. Introduction
2. Materials and Methods
2.1. Nociceptive Behavioral Tests
2.2. Open Field Behavioral Tests
2.3. Sticky-Tape Removal Tests
2.4. Measurements of Food Intake, Water Intake, and Body Weight
2.5. Immunostaining Analysis
2.6. Reverse Transcription–Polymerase Chain Reaction (RT-PCR) and Quantitative RT-PCR
2.7. Cell Cultures and Transfections
2.8. Fluorescence Intracellular Ca2+ Imaging Experiments
2.9. Spinal Cord Slice Preparation
2.10. Whole-Cell Patch Clamp Recordings
2.11. Ex Vivo Nociceptor Recordings
2.12. Compounds
2.13. Data Analysis
2.14. Data Availability
3. Results
3.1. GPR171 Is Expressed in Nociceptors
3.2. Peripheral GPR171 Activation Attenuates TRP-Specific Acute Pain
3.3. GPR171 Modulates DRG Neuronal Functions
3.4. Peripheral GPR171 Activation Attenuates Pathologic Pain
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- von Hehn, C.A.; Baron, R.; Woolf, C.J. Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 2012, 73, 638–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, G.; Hwang, S.W. Modulation of the Activities of Neuronal Ion Channels by Fatty Acid-Derived Pro-Resolvents. Front. Physiol. 2016, 7, 523. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.I.; Hwang, S.W. Depolarizing Effectors of Bradykinin Signaling in Nociceptor Excitation in Pain Perception. Biomol. Ther. 2018, 26, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, T.; Higashi, T.; Togashi, K.; Iida, T.; Segi, E.; Sugimoto, Y.; Tominaga, T.; Narumiya, S.; Tominaga, M. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol. Pain 2005, 1, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.Z.; Zhang, L.; Liu, T.; Park, J.Y.; Berta, T.; Yang, R.; Serhan, C.N.; Ji, R.R. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat. Med. 2010, 16, 592–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yudin, Y.; Rohacs, T. Inhibitory Gi/O-coupled receptors in somatosensory neurons: Potential therapeutic targets for novel analgesics. Mol. Pain 2018, 14, 1744806918763646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, I.; Aryal, D.K.; Wardman, J.H.; Gupta, A.; Gagnidze, K.; Rodriguiz, R.M.; Kumar, S.; Wetsel, W.C.; Pintar, J.E.; Fricker, L.D.; et al. GPR171 is a hypothalamic G protein-coupled receptor for BigLEN, a neuropeptide involved in feeding. Proc. Natl. Acad. Sci. USA 2013, 110, 16211–16216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, K.A.; Collins-Racie, L.A.; Colbert, M.; Duckett, M.; Golden-Fleet, M.; Kelleher, K.; Kriz, R.; LaVallie, E.R.; Merberg, D.; Spaulding, V.; et al. A genetic selection for isolating cDNAs encoding secreted proteins. Gene 1997, 198, 289–296. [Google Scholar] [CrossRef]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef] [Green Version]
- Bang, S.; Yoo, S.; Yang, T.J.; Cho, H.; Hwang, S.W. Isopentenyl pyrophosphate is a novel antinociceptive substance that inhibits TRPV3 and TRPA1 ion channels. Pain 2011, 152, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Yang, T.J.; Yoo, S.; Choi, S.I.; Lim, J.Y.; Cho, P.S.; Hwang, S.W. TRPV4-Mediated Anti-nociceptive Effect of Suberanilohydroxamic Acid on Mechanical Pain. Mol. Neurobiol. 2019, 56, 444–453. [Google Scholar] [CrossRef]
- Bang, S.; Yoo, S.; Yang, T.J.; Cho, H.; Hwang, S.W. 17(R)-resolvin D1 specifically inhibits transient receptor potential ion channel vanilloid 3 leading to peripheral antinociception. Br. J. Pharmacol. 2012, 165, 683–692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaplan, S.R.; Bach, F.W.; Pogrel, J.W.; Chung, J.M.; Yaksh, T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 1994, 53, 55–63. [Google Scholar] [CrossRef]
- Kim, Y.H.; Park, C.K.; Back, S.K.; Lee, C.J.; Hwang, S.J.; Bae, Y.C.; Na, H.S.; Kim, J.S.; Jung, S.J.; Oh, S.B. Membrane-delimited coupling of TRPV1 and mGluR5 on presynaptic terminals of nociceptive neurons. J. Neurosci. 2009, 29, 10000–10009. [Google Scholar] [CrossRef]
- Moqrich, A.; Hwang, S.W.; Earley, T.J.; Petrus, M.J.; Murray, A.N.; Spencer, K.S.; Andahazy, M.; Story, G.M.; Patapoutian, A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 2005, 307, 1468–1472. [Google Scholar] [CrossRef]
- Trevisani, M.; Siemens, J.; Materazzi, S.; Bautista, D.M.; Nassini, R.; Campi, B.; Imamachi, N.; Andrè, E.; Patacchini, R.; Cottrell, G.S.; et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. USA 2007, 104, 13519–13524. [Google Scholar] [CrossRef] [Green Version]
- Bennett, G.J.; Xie, Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Pogatzki, E.M.; Raja, S.N. A mouse model of incisional pain. Anesthesiology 2003, 99, 1023–1027. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.I.; Lee, H.R.; Sim, S.E.; Baek, J.; Yu, N.K.; Choi, J.H.; Ko, H.G.; Lee, Y.S.; Park, S.W.; Kwak, C.; et al. PI3Kgamma is required for NMDA receptor-dependent long-term depression and behavioral flexibility. Nat. Neurosci. 2011, 14, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Cheng, L.; Bourane, S.; Britz, O.; Padilla, C.; Garcia-Campmany, L.; Krashes, M.; Knowlton, W.; Velasquez, T.; Ren, X.; et al. Identification of spinal circuits transmitting and gating mechanical pain. Cell 2014, 159, 1417–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wardman, J.H.; Gomes, I.; Bobeck, E.N.; Stockert, J.A.; Kapoor, A.; Bisignano, P.; Gupta, A.; Mezei, M.; Kumar, S.; Filizola, M.; et al. Identification of a small-molecule ligand that activates the neuropeptide receptor GPR171 and increases food intake. Sci. Signal. 2016, 9, ra55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.; Lee, E.J.; Yun, S.; Choe, H.K.; Park, S.B.; Son, H.J.; Kim, K.S.; Dluzen, D.E.; Lee, I.; Hwang, O.; et al. Impact of circadian nuclear receptor REV-ERBalpha on midbrain dopamine production and mood regulation. Cell 2014, 157, 858–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bang, S.; Yoo, S.; Yang, T.J.; Cho, H.; Hwang, S.W. Nociceptive and pro-inflammatory effects of dimethylallyl pyrophosphate via TRPV4 activation. Br. J. Pharmacol. 2012, 166, 1433–1443. [Google Scholar] [CrossRef] [Green Version]
- Chiu, I.M.; Heesters, B.A.; Ghasemlou, N.; Von Hehn, C.A.; Zhao, F.; Tran, J.; Wainger, B.; Strominger, A.; Muralidharan, S.; Horswill, A.R.; et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 2013, 501, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Chatzigeorgiou, M.; Bang, S.; Hwang, S.W.; Schafer, W.R. tmc-1 encodes a sodium-sensitive channel required for salt chemosensation in C. elegans. Nature 2013, 494, 95–99. [Google Scholar] [CrossRef]
- Zimmermann, K.; Hein, A.; Hager, U.; Kaczmarek, J.S.; Turnquist, B.P.; Clapham, D.E.; Reeh, P.W. Phenotyping sensory nerve endings in vitro in the mouse. Nat. Protoc. 2009, 4, 174–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, J.H.; Liang, D.; Kido, K.; Sun, Y.; Clark, D.J.; Brennan, T.J. Increased local concentration of complement C5a contributes to incisional pain in mice. J. Neuroinflamm. 2011, 8, 80. [Google Scholar] [CrossRef] [Green Version]
- Lindfors, P.H.; Voikar, V.; Rossi, J.; Airaksinen, M.S. Deficient nonpeptidergic epidermis innervation and reduced inflammatory pain in glial cell line-derived neurotrophic factor family receptor alpha2 knock-out mice. J. Neurosci. 2006, 26, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Zylka, M.J.; Rice, F.L.; Anderson, D.J. Topographically distinct epidermal nociceptive circuits revealed by axonal tracers targeted to Mrgprd. Neuron 2005, 45, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Macpherson, L.J.; Xiao, B.; Kwan, K.Y.; Petrus, M.J.; Dubin, A.E.; Hwang, S.; Cravatt, B.; Corey, D.P.; Patapoutian, A. An ion channel essential for sensing chemical damage. J. Neurosci. 2007, 27, 11412–11415. [Google Scholar] [CrossRef]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Reznik, S.E.; Fricker, L.D. ProSAAS and prohormone convertase 1 are broadly expressed during mouse development. Brain Res. Gene Expr. Patterns 2002, 1, 135–140. [Google Scholar] [CrossRef]
- Morgan, D.J.; Mzhavia, N.; Peng, B.; Pan, H.; Devi, L.A.; Pintar, J.E. Embryonic gene expression and pro-protein processing of proSAAS during rodent development. J. Neurochem. 2005, 93, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Badheka, D.; Yudin, Y.; Borbiro, I.; Hartle, C.M.; Yazici, A.; Mirshahi, T.; Rohacs, T. Inhibition of Transient Receptor Potential Melastatin 3 ion channels by G-protein betagamma subunits. eLife 2017, 6, e26147. [Google Scholar] [CrossRef] [PubMed]
- Gorham, L.; Just, S.; Doods, H. Somatostatin 4 receptor activation modulates TRPV1 [correction of TPRV1] currents in dorsal root ganglion neurons. Neurosci. Lett. 2014, 573, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Quallo, T.; Alkhatib, O.; Gentry, C.; Andersson, D.A.; Bevan, S. G protein betagamma subunits inhibit TRPM3 ion channels in sensory neurons. eLife 2017, 6, e26138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Woolf, C.J. Pain TRPs. Neuron 2005, 46, 9–12. [Google Scholar] [CrossRef] [Green Version]
- McDermott, M.V.; Afrose, L.; Gomes, I.; Devi, L.A.; Bobeck, E.N. Opioid-induced signaling and antinociception are modulated by the recently deorphanized receptor, GPR171. J. Pharmacol. Exp. Ther. 2019, 371, 56–62. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, P.S.; Lee, H.K.; Choi, Y.I.; Choi, S.I.; Lim, J.Y.; Kim, M.; Kim, H.; Jung, S.J.; Hwang, S.W. GPR171 Activation Modulates Nociceptor Functions, Alleviating Pathologic Pain. Biomedicines 2021, 9, 256. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9030256
Cho PS, Lee HK, Choi YI, Choi SI, Lim JY, Kim M, Kim H, Jung SJ, Hwang SW. GPR171 Activation Modulates Nociceptor Functions, Alleviating Pathologic Pain. Biomedicines. 2021; 9(3):256. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9030256
Chicago/Turabian StyleCho, Pyung Sun, Han Kyu Lee, Young In Choi, Seung In Choi, Ji Yeon Lim, Minseok Kim, Hyun Kim, Sung Jun Jung, and Sun Wook Hwang. 2021. "GPR171 Activation Modulates Nociceptor Functions, Alleviating Pathologic Pain" Biomedicines 9, no. 3: 256. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9030256