Discovery through Machine Learning and Preclinical Validation of Novel Anti-Diabetic Peptides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Line
2.2. Animals
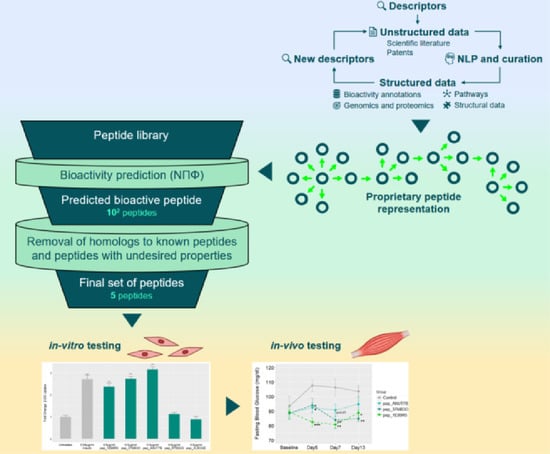
2.3. Prediction Workflow
2.4. Homology Searching and Synthesis of Predicted Peptides
2.5. Glucose Uptake Assay
2.6. GLUT4 Translocation Assay
2.7. Microarrays
2.8. Transcriptomics and Pathway Enrichment
2.9. Statistical Analyses
3. Results
3.1. Novel Peptide Prediction and Validation
3.2. Validation of Peptides in a Diabetic Mouse Model
3.3. Characteristics of Predicted Peptides
3.4. Molecular Mechanisms Modulated by pep_1E99R5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trikkalinou, A.; Papazafiropoulou, A.K.; Melidonis, A. Type 2 diabetes and quality of life. World J. Diabetes 2017, 8, 120–129. [Google Scholar] [CrossRef]
- Cho, N.; Shaw, J.; Karuranga, S.; Huang, Y.; Fernandes, J.D.R.; Ohlrogge, A.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef]
- Tahrani, A.A.; Bailey, C.J.; Del Prato, S.; Barnett, A.H. Management of type 2 diabetes: New and future developments in treatment. Lancet 2011, 378, 182–197. [Google Scholar] [CrossRef]
- Nicholson, G.; Hall, G.M. Diabetes mellitus: New drugs for a new epidemic. Br. J. Anaesth. 2011, 107, 65–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, H.B. Thoughts on the progression of type 2 diabetes drug discovery. Expert Opin. Drug Discov. 2014, 10, 107–110. [Google Scholar] [CrossRef] [Green Version]
- Turner, N.; Zeng, X.-Y.; Osborne, B.; Rogers, S.; Ye, J.-M. Repurposing Drugs to Target the Diabetes Epidemic. Trends Pharmacol. Sci. 2016, 37, 379–389. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, E. Metformin-Induced Mitochondrial Complex I Inhibition: Facts, Uncertainties, and Consequences. Front. Endocrinol. 2018, 9, 753. [Google Scholar] [CrossRef]
- Madiraju, A.K.; Erion, D.M.; Rahimi, Y.; Zhang, X.-M.; Braddock, D.T.; Albright, R.A.; Prigaro, B.J.; Wood, J.L.; Bhanot, S.; Macdonald, M.J.; et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nat. Cell Biol. 2014, 510, 542–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasri, H.; Rafieian-Kopaei, M. Metformin: Current knowledge. J. Res. Med. Sci. 2014, 19, 658–664. [Google Scholar] [PubMed]
- Hauner, H. The mode of action of thiazolidinediones. Diabetes/Metab. Res. Rev. 2002, 18, S10–S15. [Google Scholar] [CrossRef] [PubMed]
- Smith, U. Pioglitazone: Mechanism of action. Int. J. Clin. Pract. Suppl. 2001, 121, 13–18. [Google Scholar]
- Wright, E.M.; Loo, D.D.F.; Hirayama, B.A. Biology of Human Sodium Glucose Transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clar, C.; Gill, J.A.; Court, R.; Waugh, N. Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ Open 2012, 2, e001007. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V. Glucagon-like peptide-1 analogues: An overview. Indian J. Endocrinol. Metab. 2013, 17, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Cooper, M.E.; Del Prato, S. Pathophysiology and treatment of type 2 diabetes: Perspectives on the past, present, and future. Lancet 2014, 383, 1068–1083. [Google Scholar] [CrossRef] [Green Version]
- Tasyurek, H.M.; Altunbas, H.A.; Balci, M.K.; Sanlioglu, S. Incretins: Their physiology and application in the treatment of diabetes mellitus. Diabetes/Metab. Res. Rev. 2014, 30, 354–371. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Carraro, R.; Finer, N.N.; Harper, A.; Kunesova, M.; Lean, M.E.J.; Niskanen, L.; Rasmussen, M.F.; Rissanen, A.; Rössner, S.; et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. Int. J. Obes. 2011, 36, 843–854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astrup, A.; Rössner, S.; Van Gaal, L.; Rissanen, A.; Niskanen, L.; Al Hakim, M.; Madsen, J.; Rasmussen, M.F.; Lean, M.E. Effects of liraglutide in the treatment of obesity: A randomised, double-blind, placebo-controlled study. Lancet 2009, 374, 1606–1616. [Google Scholar] [CrossRef]
- Rosenstock, J.; Klaff, L.J.; Schwartz, S.; Northrup, J.; Holcombe, J.H.; Wilhelm, K.; Trautmann, M. Effects of Exenatide and Lifestyle Modification on Body Weight and Glucose Tolerance in Obese Subjects With and Without Pre-Diabetes. Diabetes Care 2010, 33, 1173–1175. [Google Scholar] [CrossRef] [Green Version]
- Zuconelli, C.R.; Brock, R.; Adjobo-Hermans, M.J. Linear Peptides in Intracellular Applications. Curr. Med. Chem. 2017, 24. [Google Scholar] [CrossRef] [PubMed]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The Future of Peptide-based Drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radermecker, R.P.; Scheen, A.J. Continuous subcutaneous insulin infusion with short-acting insulin analogues or human regular insulin: Efficacy, safety, quality of life, and cost-effectiveness. Diabetes/Metab. Res. Rev. 2004, 20, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Roxin, A.; Zheng, G. Flexible or fixed: A comparative review of linear and cyclic cancer-targeting peptides. Futur. Med. Chem. 2012, 4, 1601–1618. [Google Scholar] [CrossRef]
- Aungst, B.J.; Saitoh, H.; Burcham, D.L.; Huang, S.-M.; Mousa, S.A.; Hussain, M.A. Enhancement of the intestinal absorption of peptides and nonpeptides. J. Control. Release 1996, 41, 19–31. [Google Scholar] [CrossRef]
- Lee, A.C.-L.; Harris, J.L.; Khanna, K.K.; Hong, J.-H. A Comprehensive Review on Current Advances in Peptide Drug Development and Design. Int. J. Mol. Sci. 2019, 20, 2383. [Google Scholar] [CrossRef] [Green Version]
- Marx, V. The big challenges of big data. Nature 2013, 498, 255. [Google Scholar] [CrossRef] [Green Version]
- Schadt, E.E.; Friend, S.H.; Shaywitz, D.A. A network view of disease and compound screening. Nat. Rev. Drug Discov. 2009, 8, 286–295. [Google Scholar] [CrossRef]
- Mamoshina, P.; Vieira, A.; Putin, E.; Zhavoronkov, A. Applications of Deep Learning in Biomedicine. Mol. Pharm. 2016, 13, 1445–1454. [Google Scholar] [CrossRef]
- Rein, D.; Ternes, P.; Demin, R.; Gierke, J.; Helgason, T.; Schön, C.; Gierke, J. Artificial intelligence identified peptides modulate inflammation in healthy adults. Food Funct. 2019, 10, 6030–6041. [Google Scholar] [CrossRef] [Green Version]
- Wall, A.; Kennedy, K.; Cal, R.; Casey, R.; Holton, T.; Adelfio, A.; Khaldi, N. pep_35E7UW, a natural peptide with cutaneous anti-ageing effects discovered within the Oryza sativa proteome through machine learning. J. Dermatol. Cosmetol. 2020, 4, 109–116. [Google Scholar] [CrossRef]
- Kennedy, K.; Keogh, B.; Lopez, C.; Adelfio, A.; Molloy, B.; Kerr, A.; Wall, A.M.; Jalowicki, G.; Holton, T.A.; Khaldi, N. An Artificial Intelligence Characterised Functional Ingredient, Derived from Rice, Inhibits TNF-α and Significantly Improves Physical Strength in an Inflammaging Population. Foods 2020, 9, 1147. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, K.; Cal, R.; Casey, R.; Lopez, C.; Adelfio, A.; Molloy, B.; Wall, A.M.; Holton, T.A.; Khaldi, N. The anti-ageing effects of a natural peptide discovered by artificial intelligence. Int. J. Cosmet. Sci. 2020, 42, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Corrochano, A.R.; Cal, R.; Kennedy, K.; Wall, A.M.; Murphy, N.; Trajkovic, S.; O’Callaghan, S.; Adelfio, A.; Khaldi, N. Characterising the efficacy and bioavailability of bioactive peptides identified for attenuating muscle atrophy within a Vicia faba-derived functional ingredient. Curr. Res. Food Sci. 2021, in press. [Google Scholar]
- Guigas, B.; Sakamoto, K.; Taleux, N.; Reyna, S.M.; Musi, N.; Viollet, B.; Hue, L. Beyond AICA riboside: In search of new specific AMP-activated protein kinase activators. IUBMB Life 2009, 61, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Freymond, D.; Guignet, R.; Lhote, P.; Passaquin, A.-C.; Rüegg, U.T. Calcium homeostasis and glucose uptake of murine myotubes exposed to insulin, caffeine and 4-chloro-m -cresol. Acta Physiol. Scand. 2002, 176, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Smyth, G.K. Linear Models and Empirical Bayes Methods for Assessing Differential Expression in Microarray Experiments. Stat. Appl. Genet. Mol. Biol. 2004, 3, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.D.; Ritchie, M.E.; Smyth, G.K. Microarray background correction: Maximum likelihood estimation for the normal-exponential convolution. Biostatistics 2008, 10, 352–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. sva: Surrogate Variable Analysis, R package version 3.10; Bioconductor: New York, NY, USA, 2014. [Google Scholar]
- Wickham, H. ggplot2; Springer: New York, NY, USA, 2009. [Google Scholar]
- Di, L. Strategic Approaches to Optimizing Peptide ADME Properties. AAPS J. 2015, 17, 134–143. [Google Scholar] [CrossRef]
- Ehrenborg, E.; Krook, A. Regulation of Skeletal Muscle Physiology and Metabolism by Peroxisome Proliferator-Activated Receptor δ. Pharmacol. Rev. 2009, 61, 373–393. [Google Scholar] [CrossRef] [Green Version]
- Zisman, A.; Peroni, O.D.; Abel, E.D.; Michael, M.D.; Mauvais-Jarvis, F.; Lowell, B.B.; Wojtaszewski, J.F.; Hirshman, M.F.; Virkamaki, A.; Goodyear, L.J.; et al. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat. Med. 2000, 6, 924–928. [Google Scholar] [CrossRef]
- Vargas, E.; Podder, V.; Sepulveda, M.A.C. Physiology, Glucose Transporter Type 4 (GLUT4). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Kennedy, J.W.; Hirshman, M.F.; Gervino, E.V.; Ocel, J.V.; Forse, R.A.; Hoenig, S.J.; Aronson, D.; Goodyear, L.J.; Horton, E.S. Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes 1999, 48, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Alam, F.; Islam, A.; Khalil, I.; Gan, S.H. Metabolic Control of Type 2 Diabetes by Targeting the GLUT4 Glucose Transporter: Intervention Approaches. Curr. Pharm. Des. 2016, 22, 3034–3049. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, M.; Singh, J.; Rashed, H.; Tariq, S.; Zilahi, E.; Adeghate, E. Mechanism of the beneficial and protective effects of exenatide in diabetic rats. J. Endocrinol. 2013, 220, 291–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.-N.; Lyu, J.; Yang, X.-F.; Ji, W.-J.; Yuan, B.-X.; Chen, M.-X.; Ma, X.; Wang, B. Liraglutide ameliorates glycometabolism and insulin resistance through the upregulation of GLUT4 in diabetic KKAy mice. Int. J. Mol. Med. 2013, 32, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.W.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostawal, A.; Mocevic, E.; Kragh, N.; Xu, W. Clinical Effectiveness of Liraglutide in Type 2 Diabetes Treatment in the Real-World Setting: A Systematic Literature Review. Diabetes Ther. 2016, 7, 411–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark. Insights 2016, 11, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, S.; Gao, L.; Sun, S.; Huan, Y.; Li, C.; Wang, Y.; Guo, N.; Shen, Z. Anti-diabetic effects and mechanisms of action of a Chinese herbal medicine preparation JQ-R in vitro and in diabetic KK Ay mice. Acta Pharm. Sin. B 2017, 7, 461–469. [Google Scholar] [CrossRef]
- Angulo, P. Nonalcoholic Fatty Liver Disease. N. Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef] [Green Version]
- Franch-Nadal, J.; Caballeria, L.; Mata-Cases, M.; Mauricio, D.; Giraldez-García, C.; Mancera, J.; Goday, A.; Mundet-Tudurí, X.; Regidor, E. For the PREDAPS Study Group Fatty liver index is a predictor of incident diabetes in patients with prediabetes: The PREDAPS study. PLoS ONE 2018, 13, e0198327. [Google Scholar] [CrossRef] [Green Version]
- Fiorentino, T.V.; Andreozzi, F.; Mannino, G.C.; Pedace, E.; Perticone, M.; Sciacqua, A.; Perticone, F.; Sesti, G. One-Hour Postload Hyperglycemia Confers Higher Risk of Hepatic Steatosis to HbA1c-Defined Prediabetic Subjects. J. Clin. Endocrinol. Metab. 2016, 101, 4030–4038. [Google Scholar] [CrossRef]
- Singh, S.P.; Pati, G.K.; Misra, B.; Kar, S.K.; Panigrahi, M.K.; Meher, C.; Agrawal, O.; Rout, N.; Pattnaik, K.; Bhuyan, P.; et al. A Study of Prevalence of Diabetes and Prediabetes in Patients of Non-Alcoholic Fatty Liver Disease and the Impact of Diabetes on Liver Histology in Coastal Eastern India. J. Diabetes Mellit. 2014, 4, 290–296. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; Hoek, A.M.V.D. Establishment of a General NAFLD Scoring System for Rodent Models and Comparison to Human Liver Pathology. PLoS ONE 2014, 9, e115922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanehisa, M. From genomics to chemical genomics: New developments in KEGG. Nucleic Acids Res. 2006, 34, D354–D357. [Google Scholar] [CrossRef] [PubMed]
- Bouché, C.; Serdy, S.; Kahn, C.R.; Goldfine, A.B. The Cellular Fate of Glucose and Its Relevance in Type 2 Diabetes. Endocr. Rev. 2004, 25, 807–830. [Google Scholar] [CrossRef]
- Kitagawa, T.; Masumi, A.; Akamatsu, Y. Transforming growth factor-beta 1 stimulates glucose uptake and the expression of glucose transporter mRNA in quiescent Swiss mouse 3T3 cells. J. Biol. Chem. 1991, 266, 18066–18071. [Google Scholar] [CrossRef]
- Schwartzenberg-Bar-Yoseph, F.; Armoni, M.; Karnieli, E. The Tumor Suppressor p53 Down-Regulates Glucose Transporters GLUT1 and GLUT4 Gene Expression. Cancer Res. 2004, 64, 2627–2633. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Tzameli, I.; Bjørbaek, C.; Flier, J.S. Suppressor of Cytokine Signaling 3 Is a Physiological Regulator of Adipocyte Insulin Signaling. J. Biol. Chem. 2004, 279, 34733–34740. [Google Scholar] [CrossRef] [Green Version]
- Uhlig, T.; Kyprianou, T.; Martinelli, F.G.; Oppici, C.A.; Heiligers, D.; Hills, D.; Calvo, X.R.; Verhaert, P. The emergence of peptides in the pharmaceutical business: From exploration to exploitation. EuPA Open Proteom. 2014, 4, 58–69. [Google Scholar] [CrossRef] [Green Version]
- Tovi, A.; Eidelman, C.; Shushan, S.; Elster, S.; Hagi, A.; Ivchenko, A.; Butilca, G.M.; Zaoui, G.; Alterman, E.; Bar-Oz, L. High Purity Peptides. U.S. Patent PCT/US2008/002869, 12 September 2008. [Google Scholar]
- Paradís-Bas, M.; Tulla-Puche, J.; Albericio, F. The road to the synthesis of “difficult peptides. ” Chem. Soc. Rev. 2015, 45, 631–654. [Google Scholar] [CrossRef] [PubMed]
- Werner, H.M.; Cabalteja, C.C.; Horne, W.S. Peptide Backbone Composition and Protease Susceptibility: Impact of Modification Type, Position, and Tandem Substitution. ChemBioChem 2016, 17, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Peptide therapeutics market. Chim. Oggi-Chem. Today 2016, 34, 3. [Google Scholar]
- Lage, M.J.; Boye, K.S. The relationship between HbA1c reduction and healthcare costs among patients with type 2 diabetes: Evidence from a U.S. claims database. Curr. Med. Res. Opin. 2020, 36, 1–7. [Google Scholar] [CrossRef] [PubMed]







| Sequence | Length (Amino Acid) | Molecular Weight (Da) | Charge | Isoelectric Point | Hydrophobicity |
|---|---|---|---|---|---|
| WKDEAGKPLVK | 11 | 1270.48 | 1 | 8.5 | 45.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casey, R.; Adelfio, A.; Connolly, M.; Wall, A.; Holyer, I.; Khaldi, N. Discovery through Machine Learning and Preclinical Validation of Novel Anti-Diabetic Peptides. Biomedicines 2021, 9, 276. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9030276
Casey R, Adelfio A, Connolly M, Wall A, Holyer I, Khaldi N. Discovery through Machine Learning and Preclinical Validation of Novel Anti-Diabetic Peptides. Biomedicines. 2021; 9(3):276. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9030276
Chicago/Turabian StyleCasey, Rory, Alessandro Adelfio, Martin Connolly, Audrey Wall, Ian Holyer, and Nora Khaldi. 2021. "Discovery through Machine Learning and Preclinical Validation of Novel Anti-Diabetic Peptides" Biomedicines 9, no. 3: 276. https://0-doi-org.brum.beds.ac.uk/10.3390/biomedicines9030276