Obesity and Hypogonadism—A Narrative Review Highlighting the Need for High-Quality Data in Adolescents
Abstract
:1. Introduction
2. Methods
3. Definition and Prevalence of Male Hypogonadism
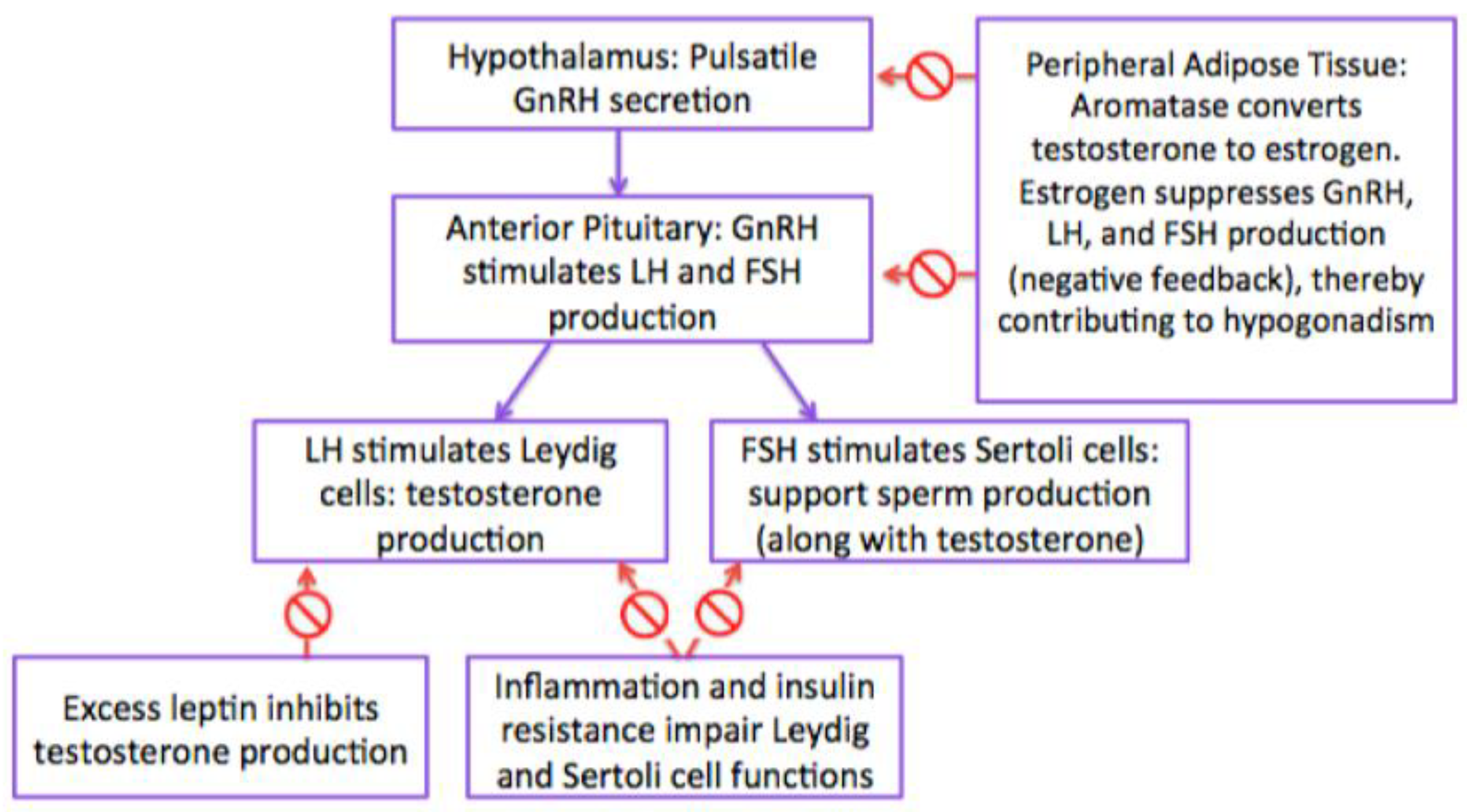
4. Pathogenesis of Hypogonadism
4.1. Hypothalamic-Pituitary-Gonadotropin (HPG) Axis
4.2. Estrogen
4.3. Gonadotropins (Follicle Stimulating Hormone and Luteinizing Hormone) and Testosterone
4.4. Sex Hormone Binding Globulin (SHBG)
4.5. INSL3
4.6. Adipokines (Leptin)
4.7. Insulin Resistance
4.8. Inflammation
4.9. Variations in Genes
4.10. Growth and Puberty in Males with Obesity
4.10.1. Timing of Puberty
4.10.2. Bone Age Advancement and Final Adult Height
5. Syndromes Associated with Obesity and Hypogonadism
6. Clinical Presentation
7. Evaluation
7.1. Diagnosis
7.2. Co-Morbidities and Complications
7.3. Consequences of Hypogonadism
7.3.1. Fatigue
7.3.2. Depression
7.3.3. Changes Associated with Low Testosterone
Bone Density
Decreased Libido
Cognition
7.4. Type 2 Diabetes and Cardiovascular Disease
7.5. Alteration of Body Composition
7.6. Quality of Life
7.7. Long-Term Fertility
7.8. Impact on the Next Generation
8. Treatment of Hypogonadism
8.1. Weight Loss
8.2. Testosterone
8.3. Clomiphene Citrate
8.4. Human Chorionic Gonadotropin (hCG)
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Key Facts. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 25 January 2019).
- WHO—10 Facts on Obesity. Available online: http://www.who.int/features/factfiles/obesity/en/ (accessed on 13 October 2018).
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity Among Adults and Youth. NCHS Data Brief. 2017, 288, 1–8. [Google Scholar]
- Raj, M.; Kumar, R.K. Obesity in children & adolescents. Indian J. Med. Res. 2010, 5, 598–607. [Google Scholar]
- High & Low Testosterone Levels: Symptoms, Signs & Side Effects. 2019, pp. 1–4. Available online: https://www.medicinenet.com/high_and_low_testosterone_levels_in_men/views.htm (accessed on 30 January 2019).
- Sigurdsson, A. Testosterone Levels, Low Testosterone Symptoms, and Testosterone Replacement Therapy. 2019, pp. 1–10. Available online: https://www.docsopinion.com/2017/10/31/testosterone-levels-symptoms-replacement-therapy/ (accessed on 30 January 2019).
- Myers, J.B.; Meacham, R.B. Androgen Replacement Therapy in the Aging Male. Rev. Urol. 2003, 5, 216–226. [Google Scholar] [PubMed]
- Seftel, A. Re: Determinants of testosterone recovery after bariatric surgery: Is it only a matter of reduction of body mass index? J. Urol. 2013, 190, 987. [Google Scholar] [CrossRef]
- Mulligan, T.; Frick, M.F.; Zuraw, Q.C.; Stemhagen, A.; McWhirter, C. Prevalence of hypogonadism in males aged at least 45 years: The HIM study. Int. J. Clin. Pract. 2006, 60, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Calderón, B.; Gómez-Martin, J.M.; Vega-Piñero, B.; Martin-Hidalgo, A.; Galindo, J.; Luque-Ramirez, M.; Escobar-Morreale, H.F.; Botella-Carretero, J.I. Prevalence of male secondary hypogonadism in moderate to severe obesity and its relationship with insulin resistance and excess body weight. Andrology 2016, 4, 62–67. [Google Scholar] [CrossRef]
- Wu, F.C.W.; Tajar, A.; Beynon, J.M.; Pye, S.R.; Silman, A.J.; Finn, J.D.; O’neil, T.W.; Bartfai, G.; Casanueva, F.F.; Forti, G.; et al. Identification of late-onset hypogonadism in middle-aged and elderly men. N. Engl. J. Med. 2010, 363, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Dhindsa, S.; Ghanim, H.; Batra, M.; Dandona, P. Hypogonadotropic hypogonadism in men with diabesity. Diabetes Care 2018, 41, 1516–1525. [Google Scholar] [CrossRef] [PubMed]
- Mogri, M.; Dhindsa, S.; Quattrin, T.; Ghanim, H.; Dandona, P. Testosterone concentrations in young pubertal and post-pubertal obese males. Clin. Endocrinol. 2013, 78, 593–599. [Google Scholar] [CrossRef]
- Chandel, A.; Dhindsa, S.; Topiwala, S.; Chaudhuri, A.; Dandona, P. Testosterone concentration in young patients with diabetes. Diabetes Care 2008, 31, 2013–2017. [Google Scholar] [CrossRef] [PubMed]
- Karakas, S.E.; Surampudi, P. Chapter three-new biomarkers to evaluate hyperandrogenic women and hypogonadal men. Adv. Clin. Chem. 2018, 86, 71–125. [Google Scholar] [PubMed]
- Liu, Y.; Ding, Z. Obesity, a serious etiologic factor for male subfertility in modern society. Reproduction 2017, R123–R131. [Google Scholar] [CrossRef] [PubMed]
- Taneli, F.; Ersoy, B.; Özhan, B.; Mehmet, C.; Ömer, Y.; Gönül, D.; Abdulkadir, G.; Taneli, C. The effect of obesity on testicular function by insulin-like factor 3, inhibin B, and leptin concentrations in obese adolescents according to pubertal stages. Clin. Biochem. 2010, 43, 1236–1240. [Google Scholar] [CrossRef] [PubMed]
- Vandewalle, S.; Taes, Y.; Fiers, T.; Van Helvoirt, M.; Debode, P.; Herregods, N.; Ernst, C.; Van Caenegem, E.; Roggen, I.; Verhelle, F.; et al. Sex steroids in relation to sexual and skeletal maturation in obese male adolescents. J. Clin. Endocrinol. Metab. 2014, 2977–2985. [Google Scholar] [CrossRef] [PubMed]
- Klein, K.O.; Larmore, K.A.; Lancey, E.D.E.; Brown, J.M.; Considine, R.V.; Hassink, S.G. effect of obesity on estradiol level, and its relationship to leptin, bone maturation, and bone mineral density in children. J. Clin. Endocrinol. Metab. 1998, 83, 3469–3475. [Google Scholar] [CrossRef] [PubMed]
- Vega, M.M.; Garach, A.M.; Fuentes, M.D.; Carlos, J.; García, F. Secondary male hypogonadism: A prevalent but overlooked comorbidity of obesity. Asian J. Androl. 2018, 20, 531–538. [Google Scholar] [CrossRef]
- Eriksson, J.; Haring, R.; Grarup, N.; Vandenput, L.; Wallaschofski, H.; Lorentzen, E.; Hansen, T.; Mellström, D.; Pedersen, O.; Nauck, M.; et al. Causal relationship between obesity and serum testosterone status in men: A bidirectional mendelian randomization analysis. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Karagiannis, A.; Harsoulis, F. Gonadal dysfunction in systemic diseases. Eur. J. Endocrinol. 2005, 152, 501–513. [Google Scholar] [CrossRef]
- Sopher, A.B.; Jean, A.M.; Zwany, S.K.; Winston, D.M.; Pomeranz, C.B.; Bell, J.J.; McMahon, D.J.; Hassoun, A.; Fennoy, I.; Oberfield, S.E. Bone age advancement in prepubertal children with obesity and premature adren.arche: Possible potentiating factors. Obesity 2011, 19, 1259–1264. [Google Scholar] [CrossRef]
- Vandewalle, S.; De Schepper, J.; Kaufman, J.M. Androgens and obesity in male adolescents. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 230–237. [Google Scholar] [CrossRef]
- Ivell, R.; Wade, J.D.; Anand-ivell, r. INSL3 as a biomarker of leydig cell functionality. Biol. Reprod. 2013, 88, 147. [Google Scholar] [CrossRef] [PubMed]
- Ferlin, A.; Foresta, C. Insulin-like factor 3: A novel circulating hormone of testicular origin in humans. Ann. N. Y. Acad. Sci. 2005, 1041, 497–505. [Google Scholar] [CrossRef]
- Cui, H.; López, M.; Rahmouni, K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat. Rev. Endocrinol. 2017, 13, 338–351. [Google Scholar] [CrossRef]
- Odle, A.K.; Akhter, N.; Syed, M.M.; Allensworth-James, M.L.; Benes, H.; Melgar Castillo, A.I.; MacNicol, M.C.; MacNicol, A.M.; Childs, G.V. Leptin regulation of gonadotrope gonadotropin-releasing hormone receptors as a metabolic checkpoint and gateway to reproductive competence. Front. Endocrinol. 2018, 8. [Google Scholar] [CrossRef]
- Ishikawa, T.; Fujioka, H.; Ishimura, T.; Takenaka, A.; Fujisawa, M. Expression of leptin and leptin receptor in the testis of fertile and infertile patients. Andrologia 2007, 39, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, R.; Patton, L.; Gambineri, A. obesity and infertility. Curr. Opin. Endocrinol. Diabetes Obes. 2007, 14, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, S.; Schöttler, P.; Engel, W.; Adham, I.M. Mouse leydig insulin-like (Ley I-L) gene: Structure and expression during testis and ovary development. Mol. Reprod. Dev. 1997, 47, 30–38. [Google Scholar] [CrossRef]
- Moriarty-kelsey, M.; Harwood, J.E.F.; Travers, S.H.; Zeitler, P.S.; Kristen, J. Insulin Resistance during Puberty. J. Pediatr. Endocrinol. Metab. 2010, 23, 1281–1287. [Google Scholar]
- Tomar, R.; Dhindsa, S.; Chaudhuri, A.; Mohanty, P.; Garg, R.; Dandona, P. Contrasting Testosterone Concentrations in Type 1 and Type 2 Diabetes. Diabetes Care 2006, 29, 1120–1122. [Google Scholar] [CrossRef]
- Giagulli, V.A.; Carbone, M.D.; Ramunni, M.I.; Licchelli, B.; De Pergola, G.; Sabbà, C.; Guastamacchia, E.; Triggiani, V. Adding liraglutide to lifestyle changes, metformin and testosterone therapy boosts erectile function in diabetic obese men with overt hypogonadism. Andrology 2015, 3, 1094–1103. [Google Scholar] [CrossRef]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef]
- Hagman, E.; Besor, O.; Hershkop, K.; Santoro, N.; Pierpont, B.; Mata, M.; Caprio, S.; Weiss, R. Relation of the degree of obesity in childhood to adipose tissue insulin resistance. Acta Diabetol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Rhee, H.; Love, T.; Harrington, D. Blood neutrophil count is associated with body mass index in adolescents with asthma. JSM Allergy Asthma 2016, 3. [Google Scholar] [CrossRef]
- Nettleship, J.E.; Pugh, P.J.; Channer, K.S.; Jones, T.; Jones, R.D. Inverse relationship between serum levels of interleukin-1βand testosterone in men with stable coronary artery disease. Horm. Metab. Res. 2007, 39, 366–371. [Google Scholar] [CrossRef]
- Wagner, I.V.; Klöting, N.; Atanassova, N.; Savchuk, I.; Spröte, C.; Kiess, W.; Söder, O.; Svechnikov, K. Prepubertal onset of obesity negatively impacts on testicular steroidogenesis in rats. Mol. Cell. Endocrinol. 2016, 437, 154–162. [Google Scholar] [CrossRef]
- Zumoff, B.; Strain, G.W.; Miller, L.K.; Rosner, W.; Senie, R.; Seres, D.S.; Rosenfield, R.S. Plasma free and non-sex-hormone-binding-globulin bound testosterone are decreased in obese men in proportion to their degree of obesity. J. Clin. Endocrinol. Metab. 1990, 71, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Cangiano, B.; Duminuco, P.; Vezzoli, V.; Guizzardi, F.; Chiodini, I.; Corona, G.; Maggi, M.; Persani, L.; Bonomi, M. Evidence for a common genetic origin of classic and milder adult-onset forms of isolated hypogonadotropic hypogonadism. J. Clin. Med. 2019, 8, 126. [Google Scholar] [CrossRef]
- Bonomi, M.; Vezzoli, V.; Krausz, C.; Guizzardi, F.; Vezzani, S.; Simoni, M. Characteristics of a nationwide cohort of patients presenting with isolated hypogonadotropic hypogonadism (IHH). Eur. J. Endocrinol. 2018, 178, 23–32. [Google Scholar] [CrossRef]
- Denzer, C.; Weibel, A.; Muche, R.; Karges, B.; Sorgo, W.; Wabitsch, M. Pediatric Highlight pubertal development in obese children and adolescents. Int. J. Obes. 2014. [Google Scholar] [CrossRef]
- He, Q.; Karlberg, J. BMI in childhood and its association with height gain, Timing of Puberty, and Final Height. Pediatr. Res. 2001, 49, 244–251. [Google Scholar] [CrossRef]
- Aksglaede, L.; Juul, A.; Olsen, L.W.; Sørensen, T.I.A. Age at puberty and the emerging obesity epidemic. PLoS ONE 2009, 4, e8450. [Google Scholar] [CrossRef] [PubMed]
- Juul, A.; Magnusdottir, S.; Scheike, T.; Prytz, S.; Skakkebæk, N.E. Age at voice break in Danish boys: Effects of pre-pubertal body mass index and secular trend. Int. J. Androl. 2007, 537–542. [Google Scholar] [CrossRef]
- Kleber, M.; Schwarz, A.; Reinehr, T. Obesity in children and adolescents: Relationship to growth, pubarche, menarche, and voice break. J. Pediatr. Endocrinol. Metab. 2011, 24, 125–130. [Google Scholar] [CrossRef]
- Wang, Y. Is obesity associated with early sexual maturation? A comparison of the Association in American boys versus girls. Pediatrics 2002, 110, 903–910. [Google Scholar] [CrossRef]
- Zhu, J.; Choa, R.E.Y.; Guo, M.H.; Plummer, L.; Buck, C.; Palmert, M.R.; Hirschhorn, J.N.; Seminara, S.B.; Chan, Y.M. A shared genetic basis for self-limited delayed puberty and idiopathic hypogonadotropic hypogonadism. J. Clin. Endocrinol. Metab. 2015, 100, E646–E654. [Google Scholar] [CrossRef]
- de Groot, C.J.; van den Berg, A.; Ballieux, B.E.P.B.; Kroon, H.M.; Rings, E.H.H.M.; Wit, J.M.; van den Akker, E.L.T. Determinants of advanced bone age in childhood obesity. Horm. Res. Paediatr. 2017, 87, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Pinhas-Hamiel, O.; Reichman, B.; Shina, A.; Derazne, E.; Tzur, D.; Yifrach, D.; Wiser, I.; Afek, A.; Shamis, A.; Tirosh, A.; et al. Sex Differences in the impact of thinness, overweight, obesity, and parental height on adolescent height. J. Adolesc. Heal. 2017, 61, 233–239. [Google Scholar] [CrossRef]
- Brener, A.; Bello, R.; Lebenthal, Y.; Yackobovitch-Gavan, M.; Phillip, M.; Shalitin, S. The impact of adolescent obesity on adult height. Horm. Res. Paediatr. 2017, 88, 237–243. [Google Scholar] [CrossRef]
- Stovitz, S.D.; Demerath, E.W.; Hannan, P.J.; Lytle, L.A.; Himes, J.H. Growing into obesity: Patterns of height growth in those who become normal weight, overweight or obese as young adults. Am. J. Hum. Biol. 2011, 23, 635–641. [Google Scholar] [CrossRef]
- Sandhu, J.; Ben-Shlomo, Y.; Cole, T.J.; Holly, J.; Smith, G.D. The impact of childhood body mass index on timing of puberty, adult stature and obesity: A follow-up study based on adolescent anthropometry recorded at Christ’s Hospital (1936–1964). Int. J. Obes. 2006, 30, 14–22. [Google Scholar] [CrossRef]
- Holmgren, A.; Niklasson, A.; Gelander, L.; Aronson, A.S.; Nierop, A.F.M.; Albertsson-Wikland, K. Insight into human pubertal growth by applying the QEPS growth model. BMC Pediatr. 2017, 17, 1–16. [Google Scholar] [CrossRef]
- Male hypogonadism-Symptoms and Causes. 2019. Available online: http://www.mayoclinic.org/diseases-conditions/male-hypogonadism/symptoms-causes/syc-20354881 (accessed on 13 October 2018).
- Ong, K.K.; Bann, D.; Wills, A.K.; Ward, K.; Adams, J.E.; Hardy, R.; Kuh, D. National Survey of Health and Development Scientific and Data Collection Team. Timing of voice breaking in males associated with growth and weight gain across the life course. J. Clin. Endocrinol. Metab. 2012, 97, 2844–2852. [Google Scholar] [CrossRef]
- Perng, W.; Gillman, M.W.; Fleisch, A.F.; Michalek, R.D.; Watkins, S.M.; Isganaitis, E.; Patti, M.E.; Oken, E. Metabolomic profiles and childhood obesity. Obesity 2014, 22, 2570–2578. [Google Scholar] [CrossRef]
- Dwyer, A.A.; Phan-hug, F.; Hauschild, M.; Elowe-Gruau, E.; Pitteloud, N. Hypogonadism in adolescence. Eur. J. Endocrinol. 2015, 173, R15–R24. [Google Scholar] [CrossRef]
- Mp, A.; Hh, A.; Ra, P. Table 4. Syndromes Associated with Hypogonadotropic Hypogonadism. GeneReviews. 2019. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/books/NBK1334/table/kms.T.syndromes_associated_with_hypogona/ (accessed on 31 January 2019).
- Bardet Biedl Syndrome Foundation. 2019. Available online: https://www.bardetbiedl.org/what-is-bbs/ (accessed on 31 January 2019).
- X-linked Adrenal Hypoplasia Congenita. Genetics Home Reference. 2019. Available online: https://ghr.nlm.nih.gov/condition/x-linked-adrenal-hypoplasia-congenita (accessed on 31 January 2019).
- Lonardo, F.; Parenti, G.; Luquetti, D.V.; Annunziata, I.; Della Monica, M.; Perone, L.; De Gregori, M.; Zuffardi, O.; Brunetti-Pierri, N.; Andria, G.; et al. Contiguous gene syndrome due to an interstitial deletion in Xp22.3 in a boy with ichthyosis, chondrodysplasia punctata, mental retardation and ADHD. Eur. J. Med. Genet. 2007, 50, 301–308. [Google Scholar] [CrossRef]
- About Prader-Willi Syndrome. 2019. Available online: https://www.fpwr.org/about-prader-willi-syndrome#definitio (accessed on 31 January 2019).
- CHARGE Syndrome. 2019. Available online: https://rarediseases.info.nih.gov/diseases/29/charge-syndrome (accessed on 31 January 2019).
- Gordon Holmes syndrome. In Genet. Home. Ref.; 2019. Available online: https://ghr.nlm.gov/condition/gordon-holmes-syndrome (accessed on 31 January 2019).
- Combined Pituitary Hormone Deficiency. In Genet. Home. Ref.; 2019. Available online: https://ghr.nlm.nih.gov/condition/combined-pituitary-hormone-deficiency (accessed on 31 January 2019).
- Barton, J.C.; Edwards, C.Q. HFE hemochromatosis. In GeneReviews; 2000. Available online: https://0-www-ncbi-nlm-nih-gov.brum.beds.ac.uk/books/NBK1440/?report=printable (accessed on 31 January 2019).
- Boehm, U.; Bouloux, P.M.; Dattani, M.T.; de Roux, N.; Dodé, C.; Dunkel, L.; Dwyer, A.A.; Giacobini, P.; Hardelin, J.P.; Juul, A.; et al. European Consensus Statement on congenital hypogonadotropic hypogonadism-pathogenesis, diagnosis and treatment. Nat. Rev. Endocrinol. 2015, 11, 547–564. [Google Scholar] [CrossRef]
- Snyder, P.J.; Bhasin, S.; Cunningham, G.R.; Matsumoto, A.M.; Stephens-Shields, A.J.; Cauley, J.A.; Gill, T.M.; Barrett-Connor, E.; Swerdloff, R.S.; Wang, C.; et al. Lessons from the testosterone trials. Endocr. Rev. 2018, 39, 369–386. [Google Scholar] [CrossRef]
- Misra, M. Bone density in the adolescent athlete. Rev. Endocr. Metab. Disord. 2008, 9, 139–144. [Google Scholar] [CrossRef]
- Greenfield, D.M.; Walters, S.J.; Coleman, R.E.; Hancock, B.W.; Eastell, R.; Davies, H.A.; Snowden, J.A.; Derogatis, L.; Shalet, S.M.; Ross, R.J. Prevalence and consequences of androgen deficiency in young male cancer survivors in a controlled cross-sectional study. J. Clin. Endocrinol. Metab. 2007, 92, 3476–3482. [Google Scholar] [CrossRef]
- Suszynski, B.M. How to Fight Fatigue From Low Testosterone. 2014, pp. 2–4. Available online: https://www.everydayhealth.com/hs/low-testosterone-guide/low-testosterone-fatigue/ (accessed on 25 January 2019).
- Turriff, A.; Levy, H.P.; Biesecker, B. Prevalence and psychosocial correlates of depressive symptoms among adolescents and adults with Klinefelter syndrome. Genet. Med. 2011, 13, 966–972. [Google Scholar] [CrossRef]
- Steidle, C.; Schwartz, S.; Jacoby, K.; Sebree, T.; Smith, T.; Bachand, R. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J. Clin. Endocrinol. Metab. 2003, 88, 2673–2681. [Google Scholar] [CrossRef]
- Almeida, O.P.; Yeap, B.B.; Hankey, G.J.; Jamrozik, K.; Flicker, L. Low free testosterone concentration as a potentially treatable cause of depressive symptoms in older men. Arch. Gen. Psychiatry 2008, 65, 283–289. [Google Scholar] [CrossRef]
- Shores, M.M.; Sloan, K.L.; Matsumoto, A.M.; Moceri, V.M.; Felker, B.; Kivlahan, D.R. Increased incidence of diagnosed depressive illness in hypogonadal older men. Arch. Gen. Psychiatry 2004, 61, 162–167. [Google Scholar] [CrossRef]
- Dallas, M.E. The Link Between Low Testosterone and Diabetes | Everyday Health. 2014. Available online: https://www.everydayhealth.com/hs/low-testosterone-guide/low-testosterone-diabetes/ (accessed on 25 January 2019).
- Rohr, U.D. The impact of testosterone imbalance on depression and women’s health. Maturitas 2002, 41, 25–46. [Google Scholar] [CrossRef]
- Mohamad, N.-V.; Soelaiman, I.-N.; Chin, K.-Y. A concise review of testosterone and bone health. Clin. Interv. Aging 2016. [Google Scholar] [CrossRef]
- Gremion, G.; Rizzoli, R.; Slosman, D.; Theintz, G.; Bonjour, J.P. Oligo-amenorrheic long-distance runners may lose more bone in spine than in femur. Med. Sci. Sports Exerc. 2001, 33, 15–21. [Google Scholar] [CrossRef]
- Singhal, V.; Bredella, M.A. Marrow adipose tissue imaging in humans. Bone 2019, 118, 69–76. [Google Scholar] [CrossRef]
- Low Testosterone and Sex Drive. 2019. Available online: https://www.webmd.com/men/how-low-testosterone-can-affect-your-sex-drive?print=true (accessed on 30 January 2019).
- Rastrelli, G.; Corona, G.; Tarocchi, M.; Mannucci, E.; Maggi, M. How to define hypogonadism? Results from a population of men consulting for sexual dysfunction. J. Endocrinol. Investig. 2016, 39, 473–484. [Google Scholar] [CrossRef]
- Chen, Y.; Niu, Y.; Xu, H.; Wang, D.; Jiang, H.; Pokhrei, G.; Wang, T.; Wang, S.; Liu, J. Testosterone undecanoate supplementation together with human chorionic gonadotropin does not impair spermatogenesis in males with isolated hypogonadotropic hypogonadism: A retrospective study Yin-Wei. Asian J. Androl. 2019, 21, 1–6. [Google Scholar] [CrossRef]
- Beauchet, O. Testosterone and cognitive function: Current clinical evidence of a relationship. Eur. J. Endocrinol. 2006, 155, 773–781. [Google Scholar] [CrossRef]
- Sisk, C.L.; Zehr, J.L. Pubertal hormones organize the adolescent brain and behavior. Front. Neuroendocrinol. 2005, 26, 163–174. [Google Scholar] [CrossRef]
- Hier, D.B.; Crowley, W.F. Spatial Ability in Androgen-deficient Men. N. Engl. J. Med. 1982, 306, 1202–1205. [Google Scholar] [CrossRef]
- Yassin, A.; Haider, A.; Haider, K.S.; Caliber, M.; Doros, G.; Saad, F.; Garvey, W.T. Testosterone therapy in men with hypogonadism prevents progression from prediabetes to type 2 diabetes: Eight-year data from a registry study. Diabetes Care 2019. [Google Scholar] [CrossRef]
- Cassimatis, D.C.; Crim, M.T.; Wenger, N.K. Low testosterone in men with cardiovascular disease or risk factors: to treat or not to treat? Curr. Treat. Opt. Cardiovasc. Med. 2016, 18, 75. [Google Scholar] [CrossRef]
- van Velzen, D.M.; Paldino, A.; Klaver, M.; Nota, N.M.; Defreyne, J.; Hovingh, G.K.; Thijs, A.; Simsek, S.; T’Sjoen, G.; den Heijer, M. Cardiometabolic effects of testosterone in transmen and estrogen plus cyproterone acetate in transwomen. J. Clin. Endocrinol. Metab. 2019, 104, 1937–1947. [Google Scholar] [CrossRef]
- Adorni, M.P.; Zimetti, F.; Cangiano, B.; Vezzoli, V.; Bernini, F.; Caruso, D.; Corsini, A.; Sirtori, C.R.; Cariboni, A.; Bonomi, M.; et al. High density lipoprotein function is reduced in patients affected by genetic or idiopathic hypogonadism. J. Clin. Endocrinol. Metab. 2019. [Google Scholar] [CrossRef]
- Saad, F.; Aversa, A.M.; Isidori, A.J.; Gooren, L. Testosterone as potential effective therapy in treatment of obesity in men with testosterone deficiency: A review. Curr. Diabetes Rev. 2012, 8, 131–143. [Google Scholar] [CrossRef]
- Palmer, N.O.; Bakos, H.W.; Fullston, T.; Lane, M. Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis 2012, 2, 253–263. [Google Scholar] [CrossRef]
- Guo, D.; Wu, W.; Tang, Q.; Qiao, S.; Chen, Y.; Chen, M.; Teng, M.; Lu, C.; Ding, H.; Xia, Y.; et al. The impact of BMI on sperm parameters and the metabolite changes of seminal plasma concomitantly. Oncotarget 2017, 8, 48619–48634. [Google Scholar] [CrossRef]
- Ramlau-Hansen, C.; Thulstrup, A.; Nohr, E.; Bonde, J.; Sorensen, T.; Olsen, J. Subfecundity in overweight and obese couples. Hum. Reprod. 2007, 22, 1634–1637. [Google Scholar] [CrossRef]
- Bakos, H.W.; Henshaw, R.C.; Mitchell, M.; Lane, M. Paternal body mass index is associated with decreased blastocyst development and reduced live birth rates following assisted reproductive technology. Fertil. Steril. 2011, 95, 1700–1704. [Google Scholar] [CrossRef]
- Colaci, D.S.; Afeiche, M.; Gaskins, A.J.; Wright, D.L.; Toth, T.L.; Tanrikut, C.; Hauser, R.; Chavarro, J.E. Men ’ s body mass index in relation to embryo quality and clinical outcomes in couples undergoing in vitro fertilization. Fertil. Steril. 2012, 98, 1193–1199. [Google Scholar] [CrossRef]
- Shrem, G.; Brudner, Y.; Atzmon, Y.; Michaeli, M.; Ellenbogen, A.; Shalom-Paz, E. The influence of obesity, smoking, and serum follicular stimulating hormone in azoospermic patients on testicular sperm extraction-intra cytoplasmic sperm injection outcomes. Medicine 2019, 98. [Google Scholar] [CrossRef]
- Arabipoor, A.; Ashrafi, M.; Hemat, M.; Zolfaghari, Z. The effects of maternal and paternal body mass index on live birth rate after intracytoplasmic sperm injection cycles. Int. J. Fertil. Steril. 2019, 13, 24–31. [Google Scholar] [CrossRef]
- Chambers, T.J.G.; Anderson, R.A. The impact of obesity on male fertility. Hormones 2015, 14, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Jaaskelainen, A.; Pussinen, J.; Nuutinen, O.; Schwab, U.; Pirkola, J.; Kolehmainen, M.; Jarvelin, M.R.; Laitinen, J. Intergenerational transmission of overweight among Finnish adolescents and their parents: A 16-year follow-up study. Int. J. Obes. 2011, 35, 1289–1294. [Google Scholar] [CrossRef]
- Fullston, T.; Palmer, N.O.; Owens, J.A.; Mitchell, M.; Bakos, H.W.; Lane, M. Diet-induced paternal obesity in the absence of diabetes diminishes the reproductive health of two subsequent generations of mice. Hum. Reprod. 2012, 27, 1391–1400. [Google Scholar] [CrossRef]
- Sermondade, N.; Faure, C.; Fezeu, L.; Shayeb, A.G.; Bonde, J.P.; Jensen, T.K.; Van Wely, M.; Cao, J.; Martini, A.C.; Eskandar, M.; et al. BMI in relation to sperm count: An updated systematic review and collaborative meta-analysis. Hum. Reprod. Update 2013, 19, 221–231. [Google Scholar] [CrossRef]
- Patro, B.; Liber, A.; Zalewski, B.; Poston, L.; Szajewska, H.; Koletzko, B. Maternal and paternal body mass index and offspring obesity: A systematic review. Ann. Nutr. Metab. 2013, 63, 32–41. [Google Scholar] [CrossRef]
- Corona, G.; Rastrelli, G.; Monami, M.; Saad, F.; Luconi, M.; Lucchese, M.; Facchiano, E.; Sforza, A.; Forti, G.; Mannucci, E.; et al. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: A systematic review and meta-analysis. Eur. J. Endocrinol. 2013, 168, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Stanik, S.; Dornfeld, L.P.; Maxwell, M.H.; Viosca, S.P.; Korenman, S.G. The effect of weight loss on reproductive hormones in obese men. J. Clin. Endocrinol. Metab. 1981, 53, 828–832. [Google Scholar] [CrossRef]
- Strain, G.W.; Zumoff, B.; Miller, L.K.; Rosner, W.; Levit, C.; Kalin, M.; Hershcopf, R.J.; Rosenfeld, R.S. Effect of massive weight loss on hypothalamic Pituitary-Gonadal function in obese men. J. Clin. Endocrinol. Metab. 1988, 66, 1019–1023. [Google Scholar] [CrossRef]
- Reis, L.O.; Favaro, W.J.; Barreiro, G.C.; de Oliveira, L.C.; Chaim, E.A.; Fregonesi, A.; Ferreira, U. Erectile dysfunction and hormonal imbalance in morbidly obese male is reversed after gastric bypass surgery: A prospective randomized controlled trial. Int. J. Androl. 2010, 33, 736–744. [Google Scholar] [CrossRef]
- Zouras, S.; Stephens, J.W.; Price, D. Obesity-related hypogonadism: A reversible condition. BMJ Case Rep. 2017. [Google Scholar] [CrossRef]
- Casulari, L.A.; Caldas, A.D.A.; Domingues Casulari Motta, L.; Lofrano-Porto, A. Effects of metformin and short-term lifestyle modification on the improvement of male hypogonadism associated with metabolic syndrome. Minerva Endocrinol. 2010, 35, 145–151. [Google Scholar]
- Krysiak, R.; Gilowski, W.; Okopien, B. The effect of metformin and metformin-testosterone combination on cardiometabolic risk factors in men with late-onset hypogonadism and impaired glucose tolerance. Exp. Clin. Endocrinol. Diabetes 2015, 123, 608–613. [Google Scholar] [CrossRef]
- Snyder, P.J.; Peachey, H.; Berlin, J.A.; Hannoush, P.; Haddad, G.; Dlewati, A.; Santanna, J.; Loh, L.; Lennow, D.A.; Holmes, J.H.; et al. Efects of testosterone replacement in hypogonadal men. J. Clin. Endocrinol. Metab. 2014, 85, 2670–2677. [Google Scholar]
- Burris, A.S.; Banks, S.M.; Carter, C.S.; Davidson, J.M.; Sherins, R.J. A long-term, prospective study of the physiologic and behavioral effects of hormone replacement in untreated hypogonadal men. J. Androl. 1992, 13, 297–304. [Google Scholar]
- Rastrelli, G.; Corona, G.; Maggi, M. Testosterone and sexual function in men. Maturitas. 2018, 11, 46–52. [Google Scholar] [CrossRef]
- Bhasin, S.; Brito, J.P.; Cunningham, G.R.; Hayes, F.J.; Hodis, H.N.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Wu, F.C.; et al. Testosterone therapy in men with hypogonadism: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2018, 103, 1715–1744. [Google Scholar] [CrossRef]
- Arslanian, S.; Chittiwat, S. Testosterone treatment in adolescents with delayed puberty: changes in body composition, protein, fat, and glucose metabolism. J. Clin. Endocrinol. Metab. 1997, 82, 3213–3220. [Google Scholar] [CrossRef]
- Arisaka, O.; Arisaka, M.; Nakayama, Y.; Fujiwara, S.; Yabuta, K. Effect of testosterone on bone density and bone metabolism in adolescent male hypogonadism. Metabolism 1995, 44, 419–423. [Google Scholar] [CrossRef]
- El Meliegy, A.; Motawi, A.; El Salam, M.A.A. Systematic review of hormone replacement therapy in the infertile man. Arab. J. Urol. 2018, 16, 140–147. [Google Scholar] [CrossRef]
- Da Ros, C.T.; Averbeck, M.A. Twenty-five milligrams of clomiphene citrate presents positive effect on treatment of male testosterone deficiency—A prospective study. Int. Braz. J. Urol. 2012, 38, 512–518. [Google Scholar] [CrossRef]
- Bendre, S.V.; Murray, P.J. Clomiphene citrate effectively increases testosterone in obese, young, hypogonadal men. Reprod. Syst. Sex. Disord. 2015, 4. [Google Scholar] [CrossRef]
- Zitzmann, M.; Nieschlag, E. Hormone substitution in male hypogonadism. Mol. Cell. Endocrinol. 2000, 161, 73–88. [Google Scholar] [CrossRef]
- Zacharin, M.R.; Warne, G.L. Treatment of hypogonadal adolescent boys with long acting subcutaneous testosterone pellets. Arch. Dis. Child. 1997, 76, 495–499. [Google Scholar] [CrossRef]
- Richman, R.A.; Kirsch, L.R. Testosterone treatment in adolescent boys with constitutional delay in growth and development. N. Engl. J. Med. 1988, 319, 1563–1567. [Google Scholar] [CrossRef]
- Crosnoe-Shipley, L.E.; Elkelany, O.O.; Cyrus, D.R.; Kim, E.D. Treatment of hypogonadotropic male hypogonadism: Case-based scenarios. World J. Nephrol. 2015, 4, 245–253. [Google Scholar] [CrossRef]
- Soares, A.H.; Horie, N.C.; Chiang, L.A.P.; Caramelli, B.; Matheus, M.G.; Campos, A.H.; Marti, L.C.; Rocha, F.A.; Mancini, M.C.; Costa, E.M.F.; et al. Effects of clomiphene citrate on male obesity-associated hypogonadism: A randomized, double-blind, placebo-controlled study. Int. J. Obes. 2018, 42, 953–963. [Google Scholar] [CrossRef]
- Taylor, F.; Levine, L. Clomiphene citrate and testosterone gel replacement therapy for male hypogonadism: Efficacy and treatment cost. J. Sex. Med. 2010, 269–276. [Google Scholar] [CrossRef]
- Moskovic, D.J.; Katz, D.J.; Akhavan, A.; Park, K.; Mulhall, J.P. Clomiphene citrate is safe and effective for long-term management of hypogonadism. BJU Int. 2012, 110, 1524–1528. [Google Scholar] [CrossRef]
- Katz, D.J.; Nabulsi, O.; Tal, R.; Mulhall, J.P. Outcomes of clomiphene citrate treatment in young hypogonadal men. BJU Int. 2012, 110, 573–578. [Google Scholar] [CrossRef]
- Yilmaz, S.; Yilmaz Sezer, N.; Gönenç, İ.M.; İlhan, S.E.; Yilmaz, E. Safety of clomiphene citrate: A literature review. Cytotechnology 2018, 70, 489–495. [Google Scholar] [CrossRef]
- Chandrapal, J.C.; Nielson, S.; Patel, D.P.; Zhang, C.; Presson, A.P.; Brant, W.O.; Myers, J.B.; Hotaling, J.M. Characterising the safety of clomiphene citrate in male patients through prostate-specific antigen, haematocrit, and testosterone levels. Br. J. Urol. Int. 2016, 118, 994–1000. [Google Scholar] [CrossRef]
- Wheeler, K.M.; Smith, R.P.; Kumar, R.A.; Setia, S.; Costabile, R.A.; Kavoussi, P.K. A comparison of secondary polycythemia in hypogonadal men treated with clomiphene citrate versus testosterone replacement: A multi-institutional study. J. Urol. 2017, 197, 1127–1131. [Google Scholar] [CrossRef]
- Cangiano, B.; Cacciatore, C.; Persani, L.; Bonomi, M. Switch to restoration therapy in a testosterone treated central hypogonadism with erythrocytosis. Endocrinol. Diabetes Metab. Case Rep. 2017. [Google Scholar] [CrossRef]
- Depenbusch, M.; von Eckardstein, S.; Simoni, M.; Nieschlag, E. Maintenance of spermatogenesis in hypogonadotropic hypogonadal men with human chorionic gonadotropin alone. Eur. J. Endocrinol. 2002, 147, 617–624. [Google Scholar] [CrossRef]
| Syndrome | Description and Main Symptoms | Associated Genes | Other Co-occurring Symptoms |
|---|---|---|---|
| Bardet-Biedl syndrome | The mutation that occurs in one of the many genes associated with this condition introduces issues with cellular communication, due to the malfunction of cilia present in cells. Symptoms involved in this disorder vary, but some of the most common are visual impairment by retinal abnormalities, obesity, and kidney malfunctions. | Mutations in at least one of 19 different genes (BBS genes): ARL6, BBIP1, BBS1, BBS2, BBS4, BBS5, BBS7, BBS10, BBS12, CEP290, IFT27, LZTFL1, MKKS, MKS1, SDCCAG8, TRIM32, TTC8, WDPCP |
|
| X-linked adrenal hypoplasia congenita | While this disorder primarily impacts males, it primarily targets adrenal glands and other endocrine tissues in the body that produce hormones to regulate functions in the body. The primary sign of this condition is adrenal insufficiency, in which the adrenal glands do not produce enough hormones. | NR0B1 |
|
| Xp22.3 contiguous gene deletion syndrome | This gene syndrome is caused by an interstitial deletion in Xp22.3. Associated symptoms include intellectual disabilities, short stature, and dysmorphic features. | Xp22.3 microdeletion |
|
| Prader-Willi Syndrome | A genetic disorder resulting from an abnormality at chromosome 15 around the time of conception. This condition impacts metabolism, growth, and cognitive function. Symptoms can change as an individual ages, but the most common involve dysfunction of the hypothalamus, growth hormone deficiency, and obesity, | Loss of paternal 15q11.2 |
|
| CHARGE syndrome | This condition is caused by mutations in the CHD7 gene which functions to make a protein involved in gene expression. This introduced glitch in gene expression causes the common symptoms that include coloboma, heart defect, atresia choanae, restricted growth, genital abnormality, and ear abnormality. | CHD7 |
|
| Gordon-Holmes Syndrome | This condition involves mutations primarily in the PNPLA6 and RNF216, which are involved in neural processes observed in synaptic connections and the release of hormones. Common symptoms involve developmental delay in puberty and neurological problems. | OTUD4 PNPLA6 RNF216 STUB1 |
|
| Combined Pituitary Hormone Deficiency | A sporadic condition that reduces the amount of different hormones produced by the pituitary gland, which may affect the development of different parts of the body. One of the primary symptoms associated with this syndrome is hypothyroidism. | HESX1 LHX3 LHX4 POU1F1 PROP1 |
|
| HFE-associated hereditary hemochromatosis | Characterized by the abnormally high absorption and storage of iron in the liver, pancreas, heart, joints, and anterior pituitary gland. This overload of iron can eventually damage tissues and organs. Some common symptoms are abdominal pain, diabetes mellitus, lethargy, and arthralgias, | HFE |
|
| Congenital Hypogonadotropic Hypogonadism | A rare condition caused by a deficiency or insensitivity to gonadotropin-releasing hormone (GnRH). This monogenic disorder is characterized through hypogonadotropic hypogonadism, in which an individual experiences incomplete or absent puberty and infertility. | GNRH, GNRH1, KISS1R, KISS1, TACR3, IL17RD, FGFR1, PROKR2 |
|
| Kallmann Syndrome | This condition is a form of hypogonadotropic hypogonadism characterized by incomplete or absent puberty and an impaired sense of smell. At puberty, most individuals do not develop secondary sexual characteristics, and potentially become infertile. | KAL1, FGFR1, FGF8, CHD7, HS6ST1, SOX10, SEMA3A, WDR11, IL17RD, PROKR2, PROK2, FEZF1 |
|
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mushannen, T.; Cortez, P.; Stanford, F.C.; Singhal, V. Obesity and Hypogonadism—A Narrative Review Highlighting the Need for High-Quality Data in Adolescents. Children 2019, 6, 63. https://0-doi-org.brum.beds.ac.uk/10.3390/children6050063
Mushannen T, Cortez P, Stanford FC, Singhal V. Obesity and Hypogonadism—A Narrative Review Highlighting the Need for High-Quality Data in Adolescents. Children. 2019; 6(5):63. https://0-doi-org.brum.beds.ac.uk/10.3390/children6050063
Chicago/Turabian StyleMushannen, Tasnim, Priscilla Cortez, Fatima Cody Stanford, and Vibha Singhal. 2019. "Obesity and Hypogonadism—A Narrative Review Highlighting the Need for High-Quality Data in Adolescents" Children 6, no. 5: 63. https://0-doi-org.brum.beds.ac.uk/10.3390/children6050063





