Observational Study of Pediatric Inpatient Pain, Nausea/Vomiting and Anxiety
Abstract
:1. Introduction
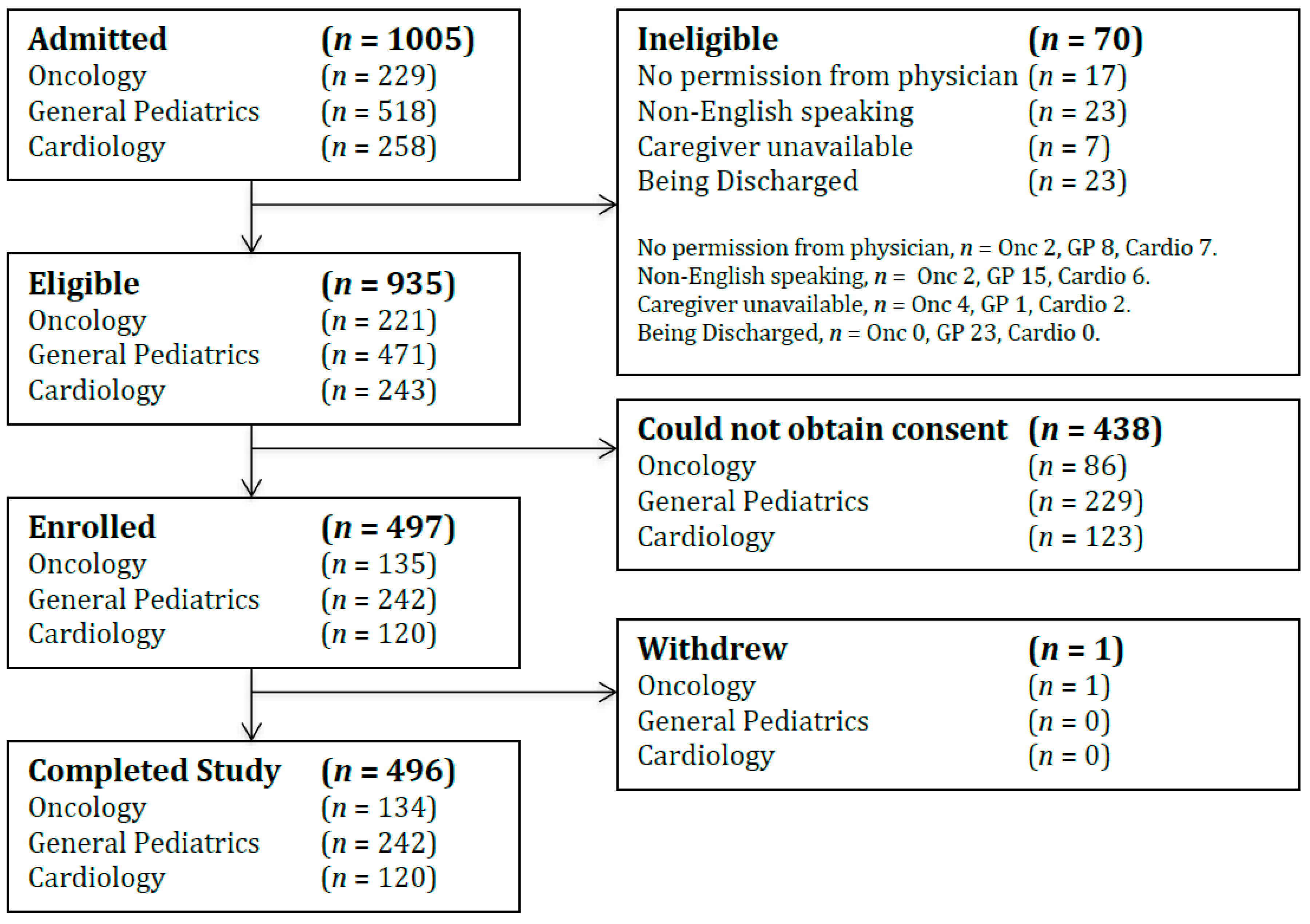
2. Materials and Methods
3. Results
3.1. Characteristics of Participants
3.2. PNVA Prevalence, Severity and Medications
3.3. Predictors of PNVA Severity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kovacic, K.; Williams, S.; Li, B.U.; Chelimsky, G.; Miranda, A. High prevalence of nausea in children with pain-associated functional gastrointestinal disorders: Are Rome criteria applicable? J. Pediatr. Gastroenterol. Nutr. 2013, 57, 311–315. [Google Scholar] [CrossRef]
- MacDonald, J.; Stuart, E.; Rodenberg, R.; Macdonald, J. Musculoskeletal low back pain in school-aged children. JAMA Pediatr. 2017, 171, 280. [Google Scholar] [CrossRef] [PubMed]
- Buchberger, B.; Huppertz, H.; Krabbe, L.; Lux, B.; Mattivi, J.T.; Siafarikas, A. Symptoms of depression and anxiety in youth with type 1 diabetes: A systematic review and meta-analysis. Psychoneuroendocrinology 2016, 70, 70–84. [Google Scholar] [CrossRef]
- Stevens, B.J.; Abbott, L.K.; Yamada, J.; Harrison, D.; Stinson, J.; Taddio, A.; Barwick, M.; Latimer, M.; Scott, S.D.; Rashotte, J.; et al. Epidemiology and management of painful procedures in children in Canadian hospitals. Can. Med. Assoc. J. 2011, 183, E403–E410. [Google Scholar] [CrossRef]
- Torio, C. Paediatric pain-related conditions impact healthcare expenditures. Evid.-Based Med. 2015, 20, 229. [Google Scholar] [CrossRef] [PubMed]
- A Cummings, E.; Reid, G.J.; Finley, G.A.; McGrath, P.J.; A Ritchie, J.; Finley, A.G. Prevalence and source of pain in pediatric inpatients. Pain 1996, 68, 25–31. [Google Scholar] [CrossRef]
- Stevens, B.J.; Harrison, D.; Rashotte, J.; Yamada, J.; Abbott, L.K.; Coburn, G.; Stinson, J.; Le May, S. Pain assessment and intensity in hospitalized children in Canada. J. Pain 2012, 13, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Stevens, B.J.; Yamada, J.; Promislow, S.; Pinard, M.; Barwick, M. Pain assessment and management after a knowledge translation booster intervention. Pediatrics 2016, 138, 20153468. [Google Scholar] [CrossRef] [PubMed]
- Gan, T.J.; Diemunsch, P.; Habib, A.S.; Kovac, A.; Kranke, P.; Meyer, T.A.; Watcha, M.; Chung, F.; Angus, S.; Apfel, C.C.; et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesthesia Analg. 2014, 118, 85–113. [Google Scholar] [CrossRef]
- Flank, J.; Robinson, P.D.; Holdsworth, M.; Phillips, R.; Portwine, C.; Gibson, P.; Maan, C.; Stefin, N.; Sung, L.; Dupuis, L.L. Guideline for the treatment of breakthrough and the prevention of refractory chemotherapy-induced nausea and vomiting in children with cancer. Pediatr. Blood Cancer 2016, 63, 1144–1151. [Google Scholar] [CrossRef]
- Heilbrunn, B.R.; Wittern, R.E.; Lee, J.B.; Pham, P.K.; Hamilton, A.H.; Nager, A.L. Reducing anxiety in the pediatric emergency department: A comparative trial. J. Emerg. Med. 2014, 47, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.M. JCAHO Pain management standards are unveiled. JAMA 2000, 284, 428–429. [Google Scholar] [CrossRef] [PubMed]
- Weisman, S.J.; Bernstein, B.; Schechter, N.L. Consequences of inadequate analgesia during painful procedures in children. Arch. Pediatr. Adolesc. Med. 1998, 152, 147–149. [Google Scholar] [CrossRef]
- Taylor, E.M.; Boyer, K.; A Campbell, F. Pain in hospitalized children: A prospective cross-sectional survey of pain prevalence, intensity, assessment and management in a Canadian pediatric teaching hospital. Pain Manag. 2008, 13, 25–32. [Google Scholar]
- Merkel, S.I.; Voepel-Lewis, T.; Shayevitz, J.R.; Malviya, S. The FLACC: A behavioural scale for scoring postoperative pain in young children. Pediatr. Nurs. 1997, 23, 293–297. [Google Scholar]
- Hicks, C.L.; Von Baeyer, C.L.; Spafford, P.A.; Van Korlaar, I.; Goodenough, B. The faces pain scale—Revised: Toward a common metric in pediatric pain measurement. Pain 2001, 93, 173–183. [Google Scholar] [CrossRef]
- Dupuis, L.L.; Taddio, A.; Kerr, E.N.; Kelly, A.; MacKeigan, L. Development and validation of the pediatric nausea assessment tool for use in children receiving antineoplastic agents. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2006, 26, 1221–1231. [Google Scholar] [CrossRef] [PubMed]
- McMurtry, C.M.; Noel, M.; Chambers, C.T.; McGrath, P.J. Children’s fear during procedural pain: Preliminary investigation of the Children’s Fear Scale. Heal. Psychol. 2011, 30, 780–788. [Google Scholar] [CrossRef]
- Ishaque, S.; Khanpour Ardestani, S.; Vohra, S. Development and validation of a pediatric anxiety faces scale. 2019. under review. [Google Scholar]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Harrison, D.; Joly, C.; Chretien, C.; Cochrane, S.; Ellis, J.; Lamontagne, C.; Vaillancourt, R. Pain prevalence in a pediatric hospital: Raising awareness during Pain Awareness Week. Pain Res. Manag. 2014, 19, e24–e30. [Google Scholar] [CrossRef]
- King, S.; Chambers, C.T.; Huguet, A.; MacNevin, R.C.; McGrath, P.J.; Parker, L.; Macdonald, A.J. The epidemiology of chronic pain in children and adolescents revisited: A systematic review. Pain 2011, 152, 2729–2738. [Google Scholar] [CrossRef]
- Jones, G.T.; Power, C.; Macfarlane, G.J. Adverse events in childhood and chronic widespread pain in adult life: Results from the 1958 British Birth Cohort Study. Pain 2009, 143, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Defenderfer, E.K.; Bauer, K.; Igler, E.; Uihlein, J.A.; Davies, W.H. The experience of pain dismissal in adolescence. Clin. J. Pain 2018, 34, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.; Jacob, E.; Hockenberry, M.J. Nausea, Pain, fatigue, and multiple symptoms in hospitalized children with cancer. Oncol. Nurs. Forum 2011, 38, 382–393. [Google Scholar] [CrossRef] [PubMed]
- A Fein, J.; Zempsky, W.T.; Cravero, J.P. Relief of pain and anxiety in pediatric patients in emergency medical systems. Pediatrics 2012, 130, 1391–1405. [Google Scholar] [CrossRef] [PubMed]
- Lamontagne, L.L.; Hepworth, J.T.; Salisbury, M.H. Anxiety and postoperative pain in children who undergo major orthopedic surgery. Appl. Nurs. 2001, 14, 119–124. [Google Scholar] [CrossRef]
- Kain, Z.N.; Mayes, L.C.; Caldwell-Andrews, A.A.; Karas, D.E.; McClain, B.C. Preoperative anxiety, postoperative pain, and behavioral recovery in young children undergoing surgery. Pediatrics 2006, 118, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Benore, E.; D’Auria, A.; Banez, G.A.; Worley, S.; Tang, A. The influence of anxiety reduction on clinical response to pediatric chronic pain rehabilitation. Clin. J. Pain 2015, 31, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Shelby, G.D.; Shirkey, K.C.; Sherman, A.L.; Beck, J.E.; Haman, K.; Shears, A.R.; Horst, S.N.; Smith, C.A.; Garber, J.; Walker, L.S. Functional abdominal pain in childhood and long-term vulnerability to anxiety disorders. Pediatrics 2013, 132, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.A.; Tran, S.T.; Jastrowski Mano, K.E.; Simpson, P.M.; Cao, Y.; Hainsworth, K.R. Predicting multiple facets of school functioning in pediatric chronic pain: Examining the direct impact of anxiety. Clin. J. Pain 2015, 31, 867–875. [Google Scholar] [CrossRef] [PubMed]
- Kain, Z.N.; Sevarino, F.; Alexander, G.M.; Pincus, S.; Mayes, L.C. Preoperative anxiety and postoperative pain in women undergoing hysterectomy. A repeated-measures design. J. Psychosom. 2000, 49, 417–422. [Google Scholar] [CrossRef]
- van den Hout, J.H.; Vlaeyen, J.W.; Houben, R.M.; Soeters, A.P.; Peters, M.L. The effects of failure feedback and pain-related fear on pain report, pain tolerance, and pain avoidance in chronic low back pain patients. Pain 2001, 92, 247–257. [Google Scholar] [CrossRef]
- Gerrits, M.M.; Van Marwijk, H.W.; Van Oppen, P.; Van Der Horst, H.; Penninx, B.W. Longitudinal association between pain, and depression and anxiety over four years. J. Psychosom. 2015, 78, 64–70. [Google Scholar] [CrossRef]
- Suls, J.; Wan, C.K. Effects of sensory and procedural information on coping with stressful medical procedures and pain: A meta-analysis. J. Consult. Clin. Psychol. 1989, 57, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Dellemijn, P.L.; Fields, H.L. Do benzodiazepines have a role in chronic pain management? Pain 1994, 57, 137–152. [Google Scholar] [CrossRef]
- Kroenke, K.; Bair, M.J.; Damush, T.M.; Wu, J.; Hoke, S.; Sutherland, J.; Tu, W. Optimized antidepressant therapy and pain self-management in primary care patients with depression and musculoskeletal pain: A randomized controlled trial. JAMA 2009, 301, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- Kroenke, K.; Bair, M.; Damush, T.; Hoke, S.; Nicholas, G.; Kempf, C.; Huffman, M.; Wu, J.; Sutherland, J. Stepped Care for Affective Disorders and Musculoskeletal Pain (SCAMP) study: Design and practical implications of an intervention for comorbid pain and depression. Gen. Hosp. Psychiatry 2007, 29, 506–517. [Google Scholar] [CrossRef]
- Dupuis, L.L.; Lu, X.; Mitchell, H.R.; Sung, L.; Devidas, M.; Mattano, L.A., Jr.; Carroll, W.L.; Winick, N.; Hunger, S.P.; Maloney, K.W.; et al. Anxiety, pain, and nausea during the treatment of standard-risk childhood acute lymphoblastic leukemia: A prospective, longitudinal study from the Children’s Oncology Group. Cancer 2016, 122, 1116. [Google Scholar] [CrossRef] [PubMed]
- Jabusch, K.M.; Lewthwaite, B.J.; Mandzuk, L.L.; Schnell-Hoehn, K.N.; Wheeler, B.J. The pain experience of inpatients in a teaching hospital: Revisiting a strategic priority. Pain Manag. Nurs. 2015, 16, 69–76. [Google Scholar] [CrossRef] [PubMed]
- McCann, M.E.; Kain, Z.N. The management of preoperative anxiety in children: An update. Anesthesia Analg. 2001, 93, 98–105. [Google Scholar] [CrossRef]
- Krauss, B. Management of acute pain and anxiety in children undergoing procedures in the emergency department. Pediatr. Emerg. Care 2001, 17, 115–122. [Google Scholar] [CrossRef]
- Vagnoli, L.; Caprilli, S.; Messeri, A. Parental presence, clowns or sedative premedication to treat preoperative anxiety in children: What could be the most promising option. Pediatr. Anesth. 2010, 20, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Golden, L.; Pagala, M.; Sukhavasi, S.; Nagpal, D.; Ahmad, A.; Mahanta, A. Giving toys to children reduces their anxiety about receiving premedication for surgery. Anesthesia Analg. 2006, 102, 1070–1072. [Google Scholar] [CrossRef]
- Patel, A.; Schieble, T.; Davidson, M. Distraction with a hand held video game reduces pediatric preoperative anciety. Pediatr. Anesth. 2006, 16, 1019–1027. [Google Scholar] [CrossRef]
- Holm, L.; Fitzmaurice, L. Emergency department waiting room stress: Can music or aromatherapy improve anxiety scores? Pediatr. Emerg. Care 2008, 24, 836–838. [Google Scholar] [CrossRef] [PubMed]
- Burks, M.L.; Brooks, E.G.; Hill, V.L.; Peters, J.I.; Wood, P.R. Assessing proxy reports: Agreement between children with asthma and their caregivers on quality of life. Ann. Allergy Asthma Immunol. 2013, 111, 14–19. [Google Scholar] [CrossRef]
| Patient Demographics | n (%) or Mean (SD) or Median (IQR) | |||
|---|---|---|---|---|
| All Departments | Oncology | General Pediatrics | Cardiology | |
| Patients | 496 | 134 | 242 | 120 |
| Age, years | 5.1 (5.7) | 8.6 (5.9) | 4.2 (5.3) | 2.6 (4.0) |
| Verbal | 185 (37.3) | 86 (64.2) | 76 (31.4) | 23 (19.2) |
| Gender | ||||
| Female | 239 (48.2) | 61 (45.5) | 129 (53.3) | 49 (40.8) |
| Ethnicity | ||||
| African | 15 (3.0) | 2 (1.5) | 9 (3.7) | 4 (3.3) |
| Arabic | 3 (0.6) | 0 | 3 (1.2) | 0 |
| Caucasian | 287 (57.9) | 68 (50.7) | 144 (59.5) | 75 (62.5) |
| Chinese | 5 (1.0) | 1 (0.7) | 2 (0.8) | 2 (1.7) |
| East Indian/Pakistani | 19 (3.8) | 2 (1.5) | 11 (4.5) | 6 (5.0) |
| Filipino | 42 (8.5) | 28 (20.9) | 10 (4.1) | 4 (3.3) |
| First Nations | 43 (8.7) | 4 (3.0) | 31 (12.8) | 8 (6.7) |
| Latin American/Mexican | 7 (1.4) | 2 (1.5) | 1 (0.4) | 4 (3.3) |
| Other | 8 (1.6) | 3 (2.2) | 3 (1.2) | 2 (1.7) |
| Identifies with multiple | 67 (13.5) | 24 (17.9) | 28 (11.6) | 15 (12.5) |
| First Admission to Hospital | 266 (53.6) | 19 (14.2) | 178 (35.9) | 69 (57.5) |
| Length of Stay, days | 3 (2–7) | 3 (2–6) | 4 (3–8) | 3 (2–9) |
| Symptom Measurements | n (%) or Mean (SD) | ||
|---|---|---|---|
| Pain (n = 496) | Nausea (n = 185) | Anxiety (n = 185) | |
| Patients Symptomatic During Admission | 256 (52.0) | 102 (55.1) | 125 (67.6) |
| Percentage of Days with Symptom | 28.2 (35.8) | 32.3 (37.8) | 46.0 (37.4) |
| Symptom Severity on Symptomatic Days (/10) | 3.0 (1.5) | 3.2 (2.1) | 3.7 (2.5) |
| Symptom | Oncology | General Pediatrics | Cardiology | p-Value |
|---|---|---|---|---|
| Mean (SD) Percentage of Days Symptomatic | ||||
| Pain | 22.7 (33.7) | 30.7 (37.5) | 26.1 (30.6) | 0.098 |
| Nausea | 29.6 (34.0) | 36.1 (39.5) | 31.2 (32.9) | 0.50 |
| Anxiety | 40.6 (41.6) | 54.9 (39.3) | 37.9 (43.2) | 0.47 |
| Mean (SD) Symptom Severity (/10) | ||||
| Pain | 2.7 (1.5) | 3.4 (1.5) | 3.3 (1.5) | 0.008 |
| Nausea | 2.8 (2.6) | 3.5 (1.6) | 4.3 (1.7) | 0.006 |
| Anxiety | 3.3 (2.3) | 4.0 (1.8) | 4.2 (4.4) | 0.11 |
| Symptom or Medication | Number (%) Patient Days | |||
|---|---|---|---|---|
| Oncology | General Pediatrics | Cardiology | p-Value | |
| Patient Days | 704 | 1451 | 1092 | |
| Reported Pain | 160 (22.7) | 445 (30.7) | 285 (26.1) | |
| Non-opioid Analgesics | 69 (9.8) | 414 (28.5) | 473 (46.0) | <0.001 |
| Opioid | 127 (18.1) | 83 (5.7) | 262 (24.0) | <0.001 |
| Reported Nausea | 208 (29.6) | 524 (36.1) | 340 (31.2) | |
| Anti-emetics | 373 (53.0) | 51 (3.5) | 370 (33.9) | <0.001 |
| Reported Anxiety | 286 (40.6) | 797 (54.9) | 414 (37.9) | |
| Anxiolytics/sedatives | 37 (5.2) | 52 (3.6) | 182 (16.7) | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schlegelmilch, M.; Punja, S.; Jou, H.; Mackie, A.S.; Conway, J.; Wilson, B.; Spavor, M.; Hartfield, D.; Vohra, S. Observational Study of Pediatric Inpatient Pain, Nausea/Vomiting and Anxiety. Children 2019, 6, 65. https://0-doi-org.brum.beds.ac.uk/10.3390/children6050065
Schlegelmilch M, Punja S, Jou H, Mackie AS, Conway J, Wilson B, Spavor M, Hartfield D, Vohra S. Observational Study of Pediatric Inpatient Pain, Nausea/Vomiting and Anxiety. Children. 2019; 6(5):65. https://0-doi-org.brum.beds.ac.uk/10.3390/children6050065
Chicago/Turabian StyleSchlegelmilch, Michael, Salima Punja, Hsing Jou, Andrew S. Mackie, Jennifer Conway, Bev Wilson, Maria Spavor, Dawn Hartfield, and Sunita Vohra. 2019. "Observational Study of Pediatric Inpatient Pain, Nausea/Vomiting and Anxiety" Children 6, no. 5: 65. https://0-doi-org.brum.beds.ac.uk/10.3390/children6050065





