Validity of Pulmonary Valve Z-Scores in Predicting Valve-Sparing Tetralogy Repairs—Systematic Review †
Abstract
:1. Introduction
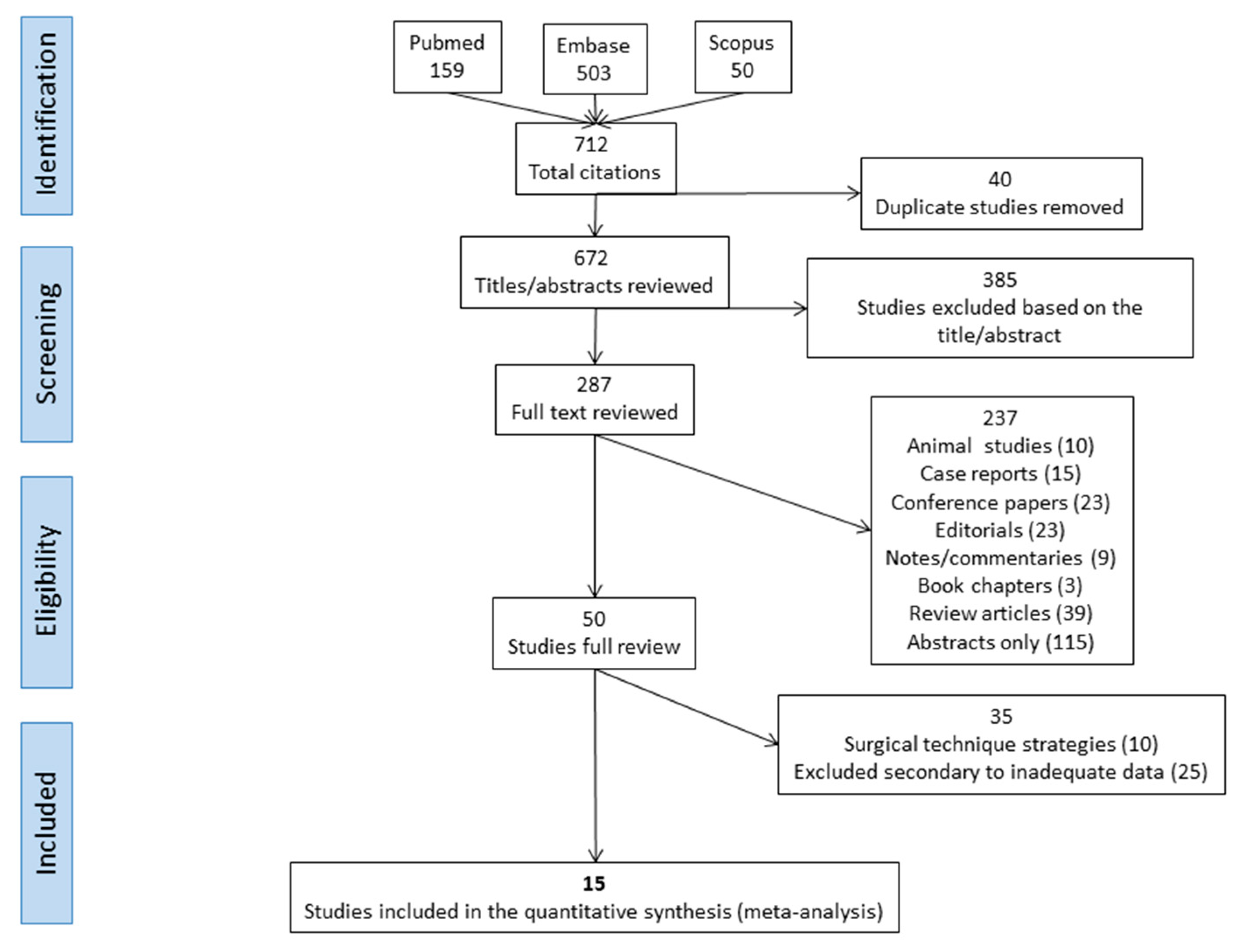
2. Materials and Methods
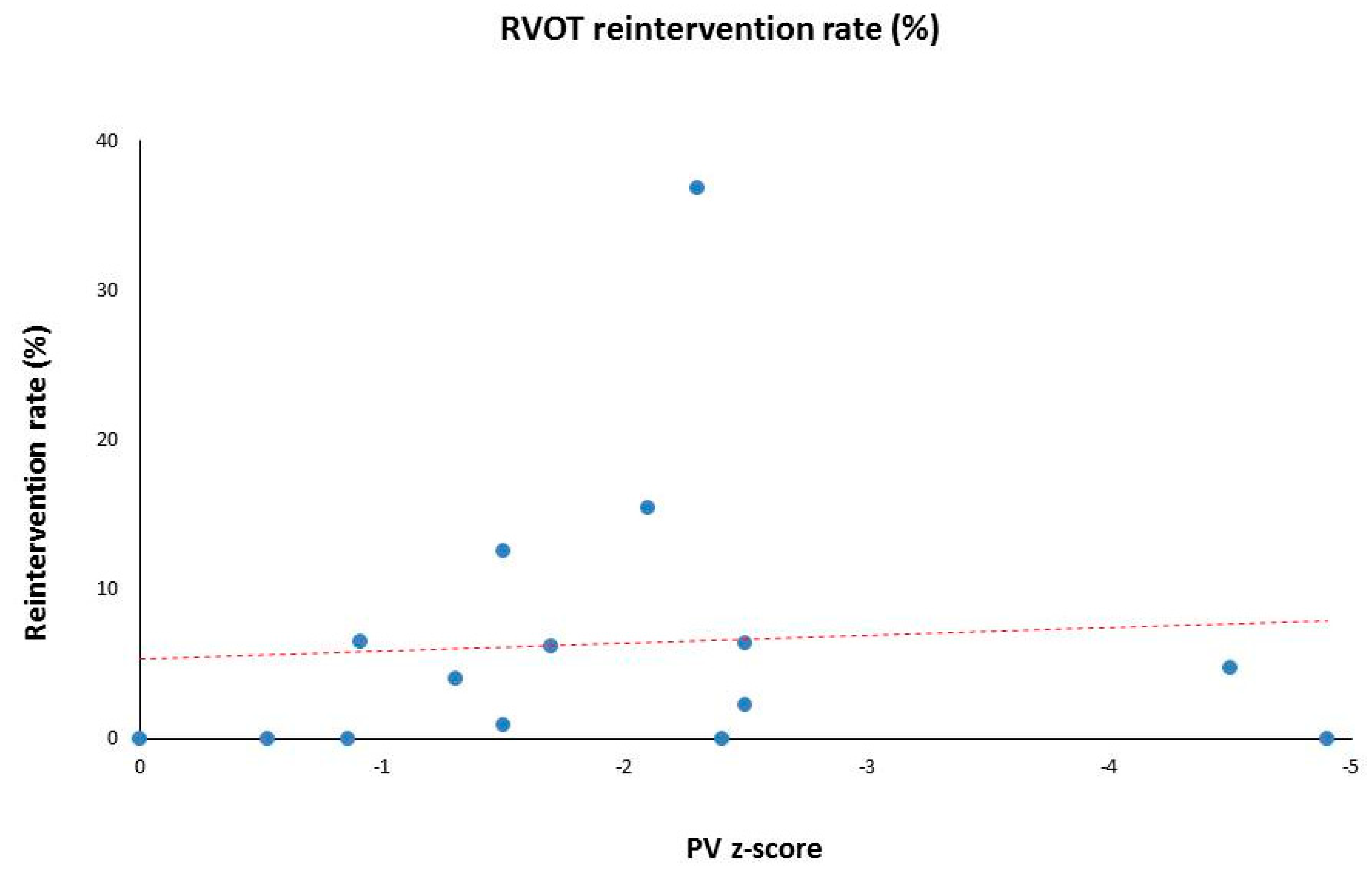
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Arafat, A.A.; Elatafy, E.E.; Elshedoudy, S.; Zalat, M.; Abdallah, N.; Elmahrouk, A. Surgical strategies protecting against right ventricular dilatation following tetralogy of Fallot repair. J. Cardiothorac. Surg. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Awori, M.N.; Mehta, N.P.; Mitema, F.O.; Kebba, N. Optimal use of z-scores to preserve the pulmonary valve annulus during repair of tetralogy of Fallot. World J. Pediatr. Congenit. Heart Surg. 2018, 9, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.D.; Backer, C.L.; Young, L.; Mavroudis, C. Tetralogy of Fallot: Results of a pulmonary valve-sparing strategy. Ann. Thorac. Surg. 2005, 80, 1431–1438. [Google Scholar] [CrossRef] [PubMed]
- Boni, L.; García, E.; Galletti, L.; Pérez, A.; Herrera, D.; Ramos, V.; Marianeschi, S.M.; Comas, J.V. Current strategies in tetralogy of Fallot repair: Pulmonary valve sparing and evolution of right ventricle/left ventricle pressures ratio. Eur. J. Cardiothorac. Surg. 2009, 35, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Hua, Z.; Li, S.; Wang, L.; Hus, S.; Wang, D. A new pulmonary valve cusp plasty technique markedly decreases transannular patch rate and improves midterm outcomes of tetralogy of Fallot. Eur. J. Cardiothorac. Surg. 2011, 40, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Bové, T.; François, K.; Van De Kerckhove, K.; Panzer, J.; De Groote, K.; De Wolf, D.; Van Nooten, G. Assessment of a right-ventricular infundibulum-sparing approach in transatrial-transpulmonary repair of tetralogy of Fallot. Eur. J. Cardiothorac. Surg. 2012, 41, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Vida, V.L.; Padalino, M.A.; Maschietto, N.; Biffanti, R.; Anderson, R.H.; Milanesi, O.; Stellin, G. The balloon dilation of the pulmonary valve during early repair of tetralogy of Fallot. Catheter Cardiovasc. Interv. 2012, 80, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Awori, M.N.; Leong, W.; Artrip, J.H.; O’Donnell, C. Tetralogy of Fallot repair: Optimal z-score use for transannular patch insertion. Eur. J. Cardiothorac. Surg. 2013, 43, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Hernandez, V.; Cardenas, I.; Martinez-Bendayan, I.; Loyola, H.; Rueda, F.; Portela, F. Valve-sparing tetralogy of Fallot repair with intraoperative dilation of the pulmonary valve. Pediatr. Cardiol. 2013, 34, 918–923. [Google Scholar] [CrossRef] [PubMed]
- Sasson, L.; Houri, S.; Raucher Sternfeld, A.; Cohen, I.; Lenczner, O.; Bove, E.L.; Kapusta, L.; Tamir, A. Right ventricular outflow tract strategies for repair of tetralogy of Fallot: effect of monocusp valve reconstruction. Eur. J. Cardiothorac. Surg. 2013, 43, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Ota, N.; Murata, M.; Tosaka, Y.; Ide, Y.; Tachi, M.; Sugimoto, A.; Sakamoto, K. Technical modification enabling pulmonary valve-sparing repair of a severely hypoplastic pulmonary annulus in patients with tetralogy of Fallot. Interact. Cardiovasc. Thorac. Surg. 2013, 16, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Hoashi, T.; Kagisaki, K.; Meng, Y.; Sakaguchi, H.; Kurosaki, K.; Shiraishi, I.; Yagihara, T.; Ichikawa, H. Long-term outcomes after definitive repair for tetralogy of Fallot with preservation of the pulmonary valve annulus. J. Thorac. Cardiovasc. Surg. 2014, 148, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Simon, B.V.; Swartz, M.F.; Egan, M.; Cholette, J.M.; Gensini, F.; Alfieris, G.M. Use of a dacron annular sparing versus limited transannular patch with nominal pulmonary annular expansion in infants with tetralogy of Fallot. Ann. Thorac. Surg. 2017, 103, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Hickey, E.; Pham-Hung, E.; Halvorsen, F.; Gritti, M.; Duong, A.; Wilder, T.; Caldarone, C.A.; Redington, A.; Van Arsdell, G. Annulus-sparing tetralogy of Fallot repair: Low risk and benefits to right ventricular geometry. Ann. Thorac. Surg. 2018, 106, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Hofferberth, S.C.; Nathan, M.; Marx, G.R.; Lu, M.; Sleeper, L.A.; Marshall, A.C.; Baird, C.W.; Mayer, J.E.; Del Nido, P.J.; Emani, S.M. Valve-sparing repair with intraoperative balloon dilation in tetralogy of Fallot: Midterm results and therapeutic implications. J. Thorac. Cardiovasc. Surg. 2018, 155, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanya, S.; Zurakowski, D.; Borisuk, M.; Kaza, A.K.; Emani, S.M.; Del Nido, P.J.; Baird, C.W. Right ventricular outflow tract reintervention after primary tetralogy of Fallot repair in neonates and young infants. J. Thorac. Cardiovasc. Surg. 2018, 155, 726–734. [Google Scholar] [CrossRef] [PubMed]
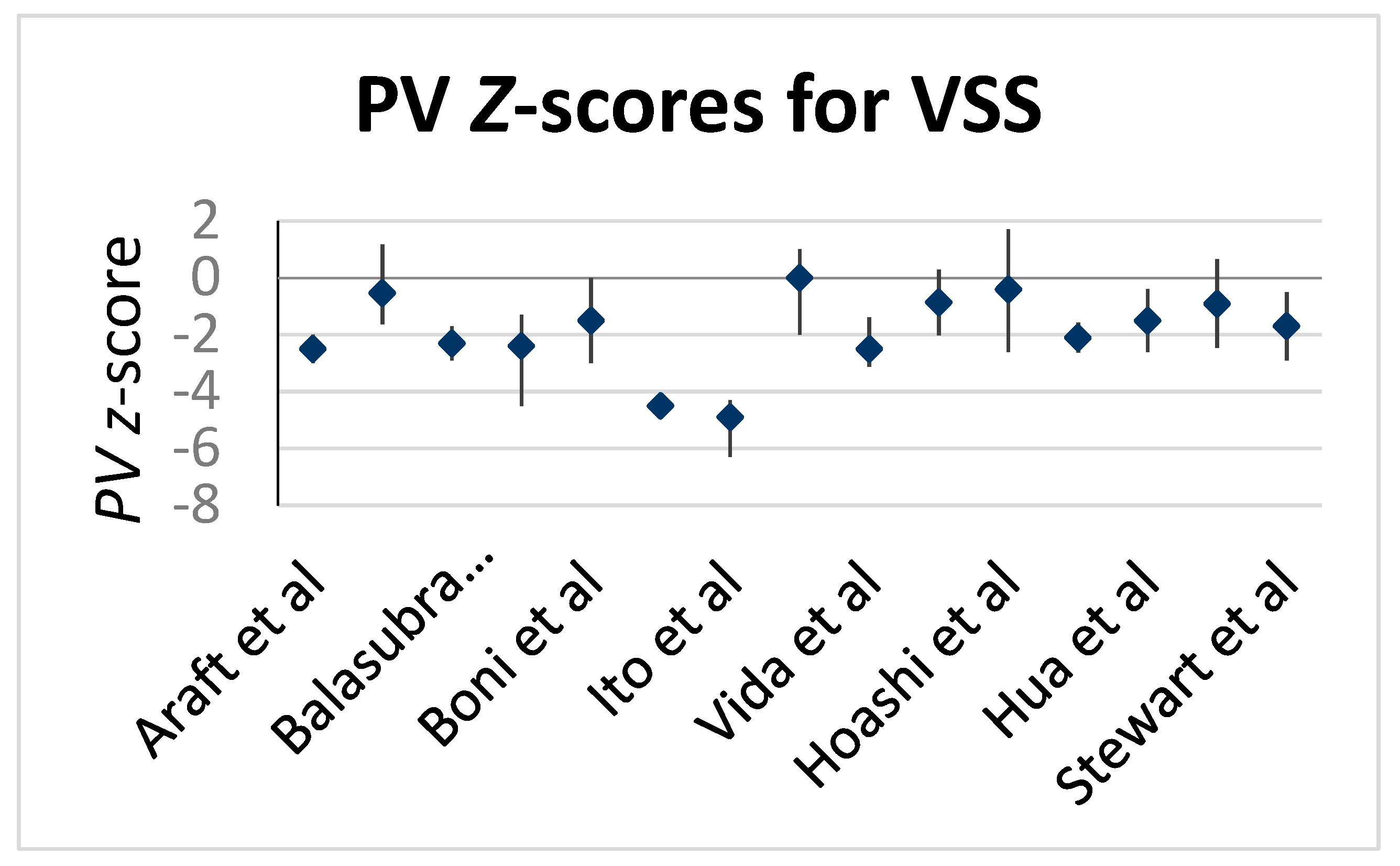
| Author, Publication Year | Patients (n) with VSS | Median Age (months) at the Time of Surgery | Median (with Range) Weight at the Time of Surgery (kg) | Pre-Op PV Z-Score Median | RVOT Re-Intervention Rate (%) | Follow-Up (y) Median | Operative Technique |
|---|---|---|---|---|---|---|---|
| Stewart, 2005 [3] | 82 | 9.4 ± 9 (mean) | 7.4 ± 5.8 (mean) | −1.7 | 6.1 | 2.8 | TA ± TP |
| Boni, 2009 [4] | 24 | 8.1 | 8.05 (5-16.5) | −1.5 | 12.5 | 2.7 | TA + TP |
| Hua, 2011 [5] | 132 | 8.1 ± 3.2 (mean) | 7.8 ± 6.2 (mean) | −1.5 | 0.9 | 2.3 | TA + TP |
| Bove, 2012 [6] | 48 | 7 | - | −0.86 | - | 7.5 | TA + TP |
| Vida, 2012 [7] | 16 | 3.1 | 5.2 (4.6–6.8) | −2.5 | 6.3 | 1.4 | TA + TP |
| Awori, 2013 [8] | 46 | 6.5 | 6.6 | −0.53 | 0 | - | TP |
| Bautista-Hernandez, 2013 [9] | 10 | 5.5 | 7.5 (4.7–47) | −2.4 | 0 | 1.8 | TI + TP |
| Sasson, 2013 [10] | 69 | 36 | 11.7 (4.3–49) | 0 | 0 | - | TA |
| Ito, 2013 [11] | 11 | 6.9 | (4.6–9.2) | −4.9 | 0 | 2.6 | TA + TP |
| Hoashi, 2014 [12] | 84 | 22.8 ± 16.8 | 9.3 ± 2.7 | −1.3 | 4 | 15.8 | TA + TP |
| Simon, 2017 [13] | 46 | 4.8 | 6 (2–10) | −0.91 | 6.5 | 7.9 | TA ± TI |
| Hickey, 2018 [14] | 296 | 5.9 | 6.8 (2.5–85) | −4.5 | 4.7 | 13.7 | TP |
| Hofferberth, 2018 [15] | 162 | 3.2 | 5.4 (4.6–6.1) | −2.1 | 15.4 | 3 | TI + TP |
| Arafat, 2018 [1] | 46 | 11 | 9 (6–16) | −2.5 | 2.2 | 3.9 | TA + TP |
| Balasubramanya, 2018 [16] | 19 | 0.5 | 3.4 (2.5–3.9) | −2.3 | 36.8 | 2.2 | - |
| Median Values | Total N = 1091 | 6.9 | 7.2 | −1.7 (0 to −4.9) | 4.5 (0–36.8) | 2.8 (1.4–15.8) |
| Author, Year of Publication | Pulmonary Valve Morphology (%) | Prior Shunt (mBT Shunt or RVOT Stent or PDA Stent) | ||
|---|---|---|---|---|
| Bicuspid | Tricuspid | Monocuspid | ||
| Stewart, 2005 [3] | 56/82 (68%) | 26/82 (32%) | - | 15 (18.3%) |
| Boni, 2009 [4] | 15/24 (62.5%) | 9/24 (37.5%) | - | 0 |
| Hua, 2011 [5] | 99/111 (89.2%) | 12/111 (10.8%) | - | 0 |
| Bove, 2012 [6] | - | - | - | 5 (10%) |
| Bautista-Hernandez, 2013 [9] | 8/10 (80%) | 2/10 (20%) | - | 0 |
| Sasson, 2013 [10] | 24.6% | - | - | 2 (2.9%) |
| Ito, 2013 [11] | 10/11 (90%) | - | - | 0 |
| Hoashi, 2014 [12] | 56/84 (66.7%) | 27/84 (32.1%) | - | 11 (13.1%) |
| Hickey, 2018 [14] | - | - | - | 13 (4.4%) |
| Hofferberth, 2018 [15] | 123 (76%) | 25 (15%) | 14 (9%) | 9 (5.6%) |
| Arafat, 2018 [1] | 26 (56.5%) | 17 (37%) | 1 (2.2%) | 8 (17.4%) |
| Total N = 63 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinha, R.; Gooty, V.; Jang, S.; Dodge-Khatami, A.; Salazar, J.
Validity of Pulmonary Valve Z-Scores in Predicting Valve-Sparing Tetralogy Repairs—Systematic Review
Sinha R, Gooty V, Jang S, Dodge-Khatami A, Salazar J.
Validity of Pulmonary Valve Z-Scores in Predicting Valve-Sparing Tetralogy Repairs—Systematic Review
Sinha, Raina, Vasu Gooty, Subin Jang, Ali Dodge-Khatami, and Jorge Salazar.
2019. "Validity of Pulmonary Valve Z-Scores in Predicting Valve-Sparing Tetralogy Repairs—Systematic Review





