An Approach to Early Detection of Metabolic Syndrome through Non-Invasive Methods in Obese Children
Abstract
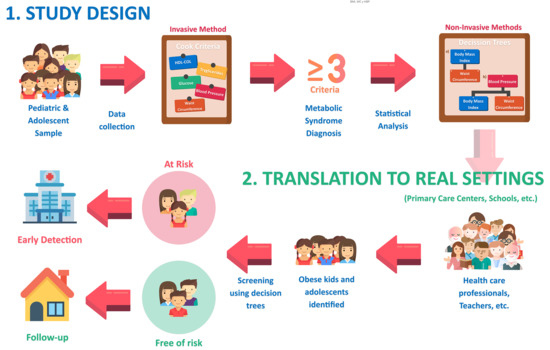
:1. Introduction
2. Materials and Methods
2.1. Design, Population, Sample
2.2. Study Variables and Measurements
- Anthropometric variables: weight (Kg), height (cm), BMI (Kg/m2), waist circumference (WC, cm), WHtR, fat-free mass (Kg), fat mass (Kg), fat mass percentage, SBP (mmHg) and DBP (mmHg).
- Analytical variables: glucose (mg/dL), Chol-HDL (mg/dL), Chol-LDL (mg/dL), triglycerides (mg/dL) and total cholesterol (mg/dL)
- Normal weight: Z-Score BMI > −2SD (standard deviation) and < +1SD;
- Overweight: Z-Score BMI > +1SD and < +2SD;
- Obesity: Z-Score BMI > +2SD.
2.3. Ethical and Legal Aspects
2.4. Statistical Analysis
- Binary logistic regression models adjusted by diverse qualitative and quantitative predictive variables. The Odds Ratio (OR) adjusted with its 95% confidence interval was determined. The goodness of fit tests (–2 log-likelihood, goodness of fit statistic, Cox and Snell R2, Nagelkerke R2 y Hosmer–Lemeshow tests) were calculated to evaluate the overall fit of the model.
- Discriminant Analysis models adjusted only by quantitative predictive variables. The coefficients for each of Fisher’s linear discriminant functions (MetS negative and MetS positive) were obtained. The Box M test was used to contrast the equalness of the matrixes for the two groups (MetS negative and MetS positive), and the Wilks Lambda test for contrasting the discriminant capacity compared with the predictive variables.
3. Results
3.1. Prevalence of MetS and Anthropometric Predictor Variables
3.2. Designing the Decision Tree to Detect MetS Based on Anthropometric Variables (Non-Invasive Method)
- -
- Model 1: based on sex (male and female) and dichotomization (according to whether the value is higher or lower than the cut-off point shown in Figure 1) of the WHtR (0.55), blood pressure (104.5/69 mmHg) and BMI (23.5 Kg/m2).
- -
- Model 2: including sex (male and female), WC, HBP according to age, sex and height, and BMI (≥23.5 Kg/m2).
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G.P. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Noncommunicable Diaseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 26 October 2020).
- Alberti, K.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.E.; Han, K.; Kang, Y.M.; Kim, S.-O.; Cho, Y.K.; Ko, K.S.; Park, J.-Y.; Lee, K.-U.; Koh, E.H. Trends in the prevalence of metabolic syndrome and its components in South Korea: Findings from the Korean National Health Insurance Service Database (2009–2013). PLoS ONE 2018, 13, e0194490. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.; Kongpakpaisarn, K.; Bohra, C. Trends in the prevalence of metabolic syndrome and its components in the United States 2007–2014. Int. J. Cardiol. 2018, 259, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Younis, A.; Younis, A.; Tzur, B.; Peled, Y.; Shlomo, N.; Goldenberg, I.; Fisman, E.Z.; Tenenbaum, A.; Klempfner, R. Metabolic syndrome is independently associated with increased 20-year mortality in patients with stable coronary artery disease. Cardiovasc. Diabetol. 2016, 15, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogden, C.L.; Carroll, M.D.; Lawman, H.G.; Fryar, C.D.; Kruszon-Moran, D.; Kit, B.K.; Flegal, K.M. Trends in Obesity Prevalence among Children and Adolescents in the United States, 1988-1994 through 2013–2014. JAMA 2016, 315, 2292–2299. [Google Scholar] [CrossRef]
- Al-Hamad, D.; Raman, V. Metabolic syndrome in children and adolescents. Transl. Pediatr. 2017, 6, 397–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.; Hong, H.; Park, S.; Kim, S.; Kang, H. Insulin Resistance and Its Association with Metabolic Syndrome in Korean Children. BioMed Res. Int. 2017, 2017, 8728017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friend, A.; Craig, L.; Turner, S. The Prevalence of Metabolic Syndrome in Children: A Systematic Review of the Literature. Metab. Syndr. Relat. Disord. 2013, 11, 71–80. [Google Scholar] [CrossRef]
- Ministerio de Salud de Chile. Encuesta Nacional de Salud 2016–2017. Available online: https://www.minsal.cl/wp-content/uploads/2018/01/2-Resultados-ENS_MINSAL_31_01_2018.pdf (accessed on 26 October 2020).
- Eyzaguirre, F.; Silva, R.; Román, R.; Palacio, A.; Cosentino, M.; Vega, V.; García, H. Prevalencia de síndrome metabólico en niños y adolescentes que consultan por obesidad. Rev. Med. Chile 2011, 139, 732–738. [Google Scholar] [CrossRef] [Green Version]
- Bustos, P.; Orias, J.; Sáez, K.; Maldonado, M.; Cuadra, L.; Asenjo, S. Impacto del Programa de manejo de la obesidad Bright Bodies aplicado a niños y adolescentes chilenos. Rev. Med. Chile 2015, 143, 1136–1143. [Google Scholar] [CrossRef] [Green Version]
- Mastroeni, S.S.D.B.S.; Mastroeni, M.F.; Ekwaru, J.P.; Setayeshgar, S.; Veugelers, P.J.; Gonçalves, M.D.C.; Rondó, P.H.D.C. Anthropometric measurements as a potential non-invasive alternative for the diagnosis of metabolic syndrome in adolescents. Arch. Endocrinol. Metab. 2019, 63, 30–39. [Google Scholar] [CrossRef]
- Romero-Saldaña, M.; Tauler, P.; Vaquero-Abellán, M.; López-González, A.-A.; Fuentes-Jiménez, F.-J.; Aguiló, A.; Álvarez-Fernández, C.; Molina-Recio, G.; Bennasar-Veny, M. Validation of a non-invasive method for the early detection of metabolic syndrome: A diagnostic accuracy test in a working population. BMJ Open 2018, 8, e020476. [Google Scholar] [CrossRef] [Green Version]
- Romero-Saldaña, M.; Fuentes-Jiménez, F.J.; Vaquero-Abellán, M.; Álvarez-Fernández, C.; Molina-Recio, G.; López-Miranda, J. New non-invasive method for early detection of metabolic syndrome in the working population. Eur. J. Cardiovasc. Nurs. 2016, 15, 549–558. [Google Scholar] [CrossRef]
- Vasan, S.K.; Thomas, N.; Christopher, S.; Geethanjali, F.S.; Paul, T.V.; Sanjeevi, C.B. Anthropometric measurements for the prediction of the metabolic syndrome: A cross-sectional study on adolescents and young adults from southern india. Heart Asia 2011, 3, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Wicklow, B.; Becker, A.; Chateau, D.; Palmer, K.; Kozyrskij, A.; Sellers, E.A.C. Comparison of anthropometric measurements in children to predict metabolic syndrome in adolescence: Analysis of prospective cohort data. Int. J. Obes. 2015, 39, 1070–1078. [Google Scholar] [CrossRef]
- Instituto Nacional de Estadística (Chile). Censo 2017. Available online: https://www.censo2017.cl (accessed on 26 October 2020).
- Cook, S.; Weitzman, M.; Auinger, P.; Nguyen, M.; Dietz, W.H. Prevalence of a metabolic syndrome phenotype in adolescents: Findings from the third National Health and Nutrition Examination Survey, 1988−1994. Arch. Pediatr. Adolesc. Med. 2003, 157, 821–827. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2019. Diabetes Care 2018, 42 (Suppl. 1), S13–S28. [Google Scholar] [CrossRef] [Green Version]
- Callaway, C.; Chumlea, W.; Bouchard, C.; Himes, J.; Lohman, T.; Martin, A.; Mitchell, C.D.; Mueller, W.H. Circumferences. In Anthropometric Standardization Reference Manual; Lohman, T.G., Roche, A., Martorell, R., Eds.; Human Kinetics Books: Champaign, IL, USA, 1991; pp. 44–45. [Google Scholar]
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.F.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Organización Mundial de la Salud. IMC Para Edad de Entre 5 y 19 Años. Available online: https://www.who.int/growthref/who2007_bmi_for_age/en/ (accessed on 26 October 2020).
- Ministerio de Salud. Orientaciones Técnicas Para el Control de Salud Integral de Adolescentes; Control Joven Sano 2014; Ministerio de Salud: Santiago, Chile, 2013. [Google Scholar]
- Fernández, J.R.; Redden, D.T.; Pietrobelli, A.; Allison, D.B. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J. Pediatr. 2004, 145, 439–444. [Google Scholar] [CrossRef]
- Mardones, F.; Araniz, P.; Barja, S.; Giadach, C.; Villarroel, L.; Domínguez, A. Estado nutricional, síndrome metabólico y resistencia a la insulina en niños de Santiago, Chile. Nutr. Hosp. 2013, 28, 1999–2005. [Google Scholar]
- Ahrens, W.; Moreno, L.; Mårild, S.; Molnár, D.; Siani, A.; de Henauw, S.; Böhmann, J.; Günther, K.; Hadjigeorgiou, C.; Iacoviello, L.; et al. Metabolic syndrome in young children: Definitions and results of the IDEFICS study. Int. J. Obes. 2014, 38 (Suppl. 2), S4–S14. [Google Scholar] [CrossRef] [Green Version]
- Pierlot, R.; Cuevas-Romero, E.; Rodríguez-Antolín, J.; Méndez-Hernández, P.; Martínez-Gómez, M. Prevalencia de síndrome metabólico en niños y adolescentes de américa. TIP 2017, 20, 40–49. [Google Scholar] [CrossRef]
- Gepstein, V.; Weiss, R. Obesity as the Main Risk Factor for Metabolic Syndrome in Children. Front. Endocrinol. 2019, 10, 568. [Google Scholar] [CrossRef]
- Sapunar, J.; Aguilar-Farías, N.; Navarro, J.; Araneda, G.; Chandia-Poblete, D.; Manríquez, V.; Brito, R.; Cerda, A. High prevalence of overweight, obesity, insulin resistance and metabolic syndrome in rural children and adolescents. Rev. Med. Chile 2018, 146, 978–986. [Google Scholar] [CrossRef] [Green Version]
- Radetti, G.; Fanolla, A.; Grugni, G.; Lupi, F.; Sartorio, A. Indexes of adiposity and body composition in the prediction of metabolic syndrome in obese children and adolescents: Which is the best? Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1189–1196. [Google Scholar] [CrossRef]
- Lee, K. Comparison of Body Mass Index Percentiles to Detect Metabolic Syndrome Using the Korean, United States Centers for Disease Control and Prevention, and World Health Organization References in Korean Children Aged 10–16 Years. Metab. Syndr. Relat. Disord. 2019, 17, 210–216. [Google Scholar] [CrossRef]
- Jung, C.; Fischer, N.; Fritzenwanger, M.; Figulla, H.R. Anthropometric indices as predictors of the metabolic syndrome and its components in adolescents. Pediatr. Int. 2010, 52, 402–409. [Google Scholar] [CrossRef]
- De Oliveira, R.G.; Guedes, D.P. Performance of anthropometric indicators as predictors of metabolic syndrome in Brazilian adolescents. BMC Pediatr. 2018, 18, 33. [Google Scholar] [CrossRef] [Green Version]
- Nambiar, S.; Truby, H.; Davies, P.S.; Baxter, K. Use of the waist-height ratio to predict metabolic syndrome in obese children and adolescents. J. Paediatr. Child Health 2013, 49, E281–E287. [Google Scholar] [CrossRef]
- Sangrador, C.O.; Ochoa-Brezmes, J. Waist-to-height ratio as a risk marker for metabolic syndrome in childhood. A meta-analysis. Pediatr. Obes. 2018, 13, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.; Wong, M.; Khalechelvam, P.; Tam, W. Waist-to-height ratio, body mass index and waist circumference for screening paediatric cardio-metabolic risk factors: A meta-analysis. Obes. Rev. 2016, 17, 1258–1275. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.; Ferrari, N. Metabolic Syndrome in Children and Adolescents. Visc. Med. 2016, 32, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Aristizabal, J.C.; Estrada-Restrepo, A.; Barona, J. Waist-to-height ratio may be an alternative tool to the body mass index for identifying Colombian adolescents with cardiometabolic risk factors. Nutr. Hosp. 2019, 36, 96–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perona, J.; Schmidt, J.; Ramírez-Vélez, R.; Correa-Rodríguez, M.; Fernández-Aparicio, Á.; González-Jiménez, E. Waist circumference and abdominal volume index are the strongest anthropometric discriminators of metabolic syndrome in Spanish adolescents. Eur. J. Clin. Investig. 2018, 49, e13060. [Google Scholar] [CrossRef]
- Pacheco, L.S.; Blanco, E.; Burrows, R.; Reyes, M.; Lozoff, B.; Gahagan, S. Early Onset Obesity and Risk of Metabolic Syndrome Among Chilean Adolescents. Prev. Chronic Dis. 2017, 14, 170132. [Google Scholar] [CrossRef] [Green Version]
- Chiarelli, F.; Mohn, A. Early diagnosis of metabolic syndrome in children. Lancet Child Adolesc. Health 2017, 1, 86–88. [Google Scholar] [CrossRef]
- Ahadi, Z.; Bahreynian, M.; Qorbani, M.; Heshmat, R.; Motlagh, M.E.; Shafiee, G.; Gorabi, A.M.; Ziaodini, H.; Taheri, M.; Aminaei, T.; et al. Association of anthropometric measures and cardio-metabolic risk factors in normal-weight children and adolescents: The CASPIAN-V study. J. Pediatr. Endocrinol. Metab. 2018, 31, 847–854. [Google Scholar] [CrossRef]
- De Quadros, T.M.B.; Gordia, A.P.; Andaki, A.C.R.; Mendes, E.L.; Mota, J.; Silva, L.R. Utility of anthropometric indicators to screen for clustered cardiometabolic risk factors in children and adolescents. J. Pediatr. Endocrinol. Metab. 2019, 32, 49–55. [Google Scholar] [CrossRef]
- Ataie-Jafari, A.; Namazi, N.; Djalalinia, S.; Chaghamirzayi, P.; Abdar, M.E.; Zadehe, S.S.; Asayesh, H.; Zarei, M.; Gorabi, A.M.; Mansourian, M.; et al. Neck circumference and its association with cardiometabolic risk factors: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2018, 10, 72. [Google Scholar] [CrossRef]
- Koskinen, J.; Magnussen, C.G.; Sinaiko, A.; Woo, J.; Urbina, E.; Jacobs, D.R.; Steinberger, J.; Prineas, R.; Sabin, M.A.; Burns, T.; et al. Childhood Age and Associations Between Childhood Metabolic Syndrome and Adult Risk for Metabolic Syndrome, Type 2 Diabetes Mellitus and Carotid Intima Media Thickness: The International Childhood Cardiovascular Cohort Consortium. J. Am. Heart Assoc. 2017, 6, e005632. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Raw Regression (No Adjusted) | Adjusted Regression | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variables | Total (n = 221) | Presence (n = 59) | Absence (n = 162) | OR | 95% CI | p | OR | 95% CI | p |
| Mean or n (SD or %) | Mean or n (SD or %) | Mean or n (SD or %) | |||||||
| Sex | |||||||||
| Women | 111 (51.2) | 38 (64.4) | 73 (45.1) | 2.206 | 1.191–4.086 | <0.05 | 3.488 | 1.586–7.674 | <0.01 |
| Men | 110 (49.8) | 21 (35.6) | 89 (54.9) | 1 | 1 | 1 | 1 | ||
| Age (years) | 9.1 (1.3) | 9.3 (1.2) | 9 (1.3) | 1.217 | 0.949–1.559 | NS | |||
| Weight (Kg) | 41.2 (10.8) | 49.2 (9) | 38.3 (9.9) | 1.124 | 1.081–1.168 | <0.001 | |||
| Height (cm) | 135.2 (9) | 138.2 (8.6) | 134.1 (8.9) | 1.057 | 1.019–1.097 | <0.01 | |||
| WC (cm) | 74.6 (11.5) | 83.6 (10.8) | 71.3 (10.8) | 1.131 | 1.086–1.177 | <0.001 | |||
| WHtR > 0.55 | 128 (57.9) | 55 (93.2) | 73 (45.1) | 16.764 | 5.801–48.441 | <0.001 | |||
| BMI (Kg/m2) | 22.2 (4.2) | 25.6 (3.1) | 21 (3.8) | 1.435 | 1.276–1.613 | <0.001 | 1.380 | 1.219–1.563 | <0.001 |
| Overweight/Obesity | 148 (67) | 57 (96.6) | 91 (56.2) | 22.236 | 5.249–94.206 | <0.001 | |||
| BF (%) | 30.1 (9.4) | 36.6 (6) | 27.7 (9.4) | 1.139 | 1.087–1.193 | <0.001 | |||
| FM (Kg) | 13.4 (6.7) | 18.3 (5.7) | 11.5 (6.2) | 1.194 | 1.124–1.268 | <0.001 | |||
| FFM (Kg) | 28.4 (5.1) | 31.1 (5.1) | 27.4 (4.7) | 1.173 | 1.093–1.258 | <0.001 | |||
| SBP (mmHg) | 101.5 (12) | 108.7 (12) | 98.9 (10.9) | 1.086 | 1.051–1.121 | <0.001 | 1.059 | 1.022–1.098 | <0.001 |
| DBP (mmHg) | 66 (10.8) | 71.1 (10.7) | 64.1 (10.2) | 1.073 | 1.037–1.109 | <0.001 | |||
| Glucose (mg/dL) | 88.2 (8.5) | 88.6 (10) | 88.1 (8) | 1.006 | 0.972–1.042 | NS | |||
| CholT (mg/dL) | 181.7 (33.1) | 188.2 (36.4) | 179.3 (31.6) | 1.008 | 0.999–1.017 | NS | |||
| Chol-HDL (mg/dL) | 50.5 (11.6) | 40.4 (10.2) | 54.2 (9.8) | 0.859 | 0.821–0.899 | <0.001 | |||
| Chol-LDL (mg/dL) | 108.2 (28.8) | 113.3 (32.9) | 106.3 (27.1) | 1.008 | 0.998–1.019 | NS | |||
| Triglycerides (mg/dL) | 122.9 (73.8) | 186 (93.1) | 99.9 (48.1) | 1.020 | 1.014–1.026 | <0.001 | |||
| Model 1 | ||||
|---|---|---|---|---|
| Variable | Coefficient | OR | 95% CI | p |
| Sex (Woman) | 1.114 | 3.047 | 1.392–6.669 | <0.01 |
| WHtR ≥ 0.55 | 1.714 | 5.551 | 1.996–15.442 | <0.01 |
| BP ≥ 104.5/69 | 1.448 | 4.253 | 1.743–10.375 | <0.01 |
| BMI ≥ 23.5 | 1.411 | 4.544 | 1.120–14.999 | <0.05 |
| Model 2 | ||||
| Sex | 1.263 | 3.535 | 1.511–8.273 | <0.01 |
| WC | 0.076 | 1.079 | 1.020–1.142 | <0.01 |
| HBP * | 1.941 | 6.964 | 3.012–16.104 | <0.001 |
| BMI ≥ 23.5 | 1.235 | 3.443 | 1.070–11.081 | <0.05 |
| Sensitivity, Specificity and Validity Index for the Predictive MetS Model | ||||
| Model 1 | Model 2 | |||
| Sensitivity | 45.8% | 66.1% | ||
| Specificity | 95.1% | 90.7% | ||
| Validity index | 81.9% | 84.2% | ||
| R2 Nagelkerke | 0.455 | 0.511 | ||
| R2 Cox-Snell | 0.313 | 0.350 | ||
| Hosmer-Lemeshow (p) | 0.734 | 0.417 | ||
| Variable | MetS [No] Coefficient | MetS [Yes] Coefficient | p |
|---|---|---|---|
| BMI | 0.960 | 1.251 | <0.001 |
| SBP | 0.674 | 0.721 | <0.001 |
| Constant | −44.106 | −55.778 | - |
| Sensitivity, Specificity and Validity Index for the Predictive MetS Model | |||
| Sensitivity | 81.4% | ||
| Specificity | 75.3% | ||
| Validity index | 76.9% | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina-Luque, R.; Ulloa, N.; Gleisner, A.; Zilic, M.; Romero-Saldaña, M.; Molina-Recio, G. An Approach to Early Detection of Metabolic Syndrome through Non-Invasive Methods in Obese Children. Children 2020, 7, 304. https://0-doi-org.brum.beds.ac.uk/10.3390/children7120304
Molina-Luque R, Ulloa N, Gleisner A, Zilic M, Romero-Saldaña M, Molina-Recio G. An Approach to Early Detection of Metabolic Syndrome through Non-Invasive Methods in Obese Children. Children. 2020; 7(12):304. https://0-doi-org.brum.beds.ac.uk/10.3390/children7120304
Chicago/Turabian StyleMolina-Luque, Rafael, Natalia Ulloa, Andrea Gleisner, Martin Zilic, Manuel Romero-Saldaña, and Guillermo Molina-Recio. 2020. "An Approach to Early Detection of Metabolic Syndrome through Non-Invasive Methods in Obese Children" Children 7, no. 12: 304. https://0-doi-org.brum.beds.ac.uk/10.3390/children7120304