Multi-System Inflammatory Syndrome in Children (MIS-C) Following SARS-CoV-2 Infection: Review of Clinical Presentation, Hypothetical Pathogenesis, and Proposed Management
Abstract
:1. Introduction
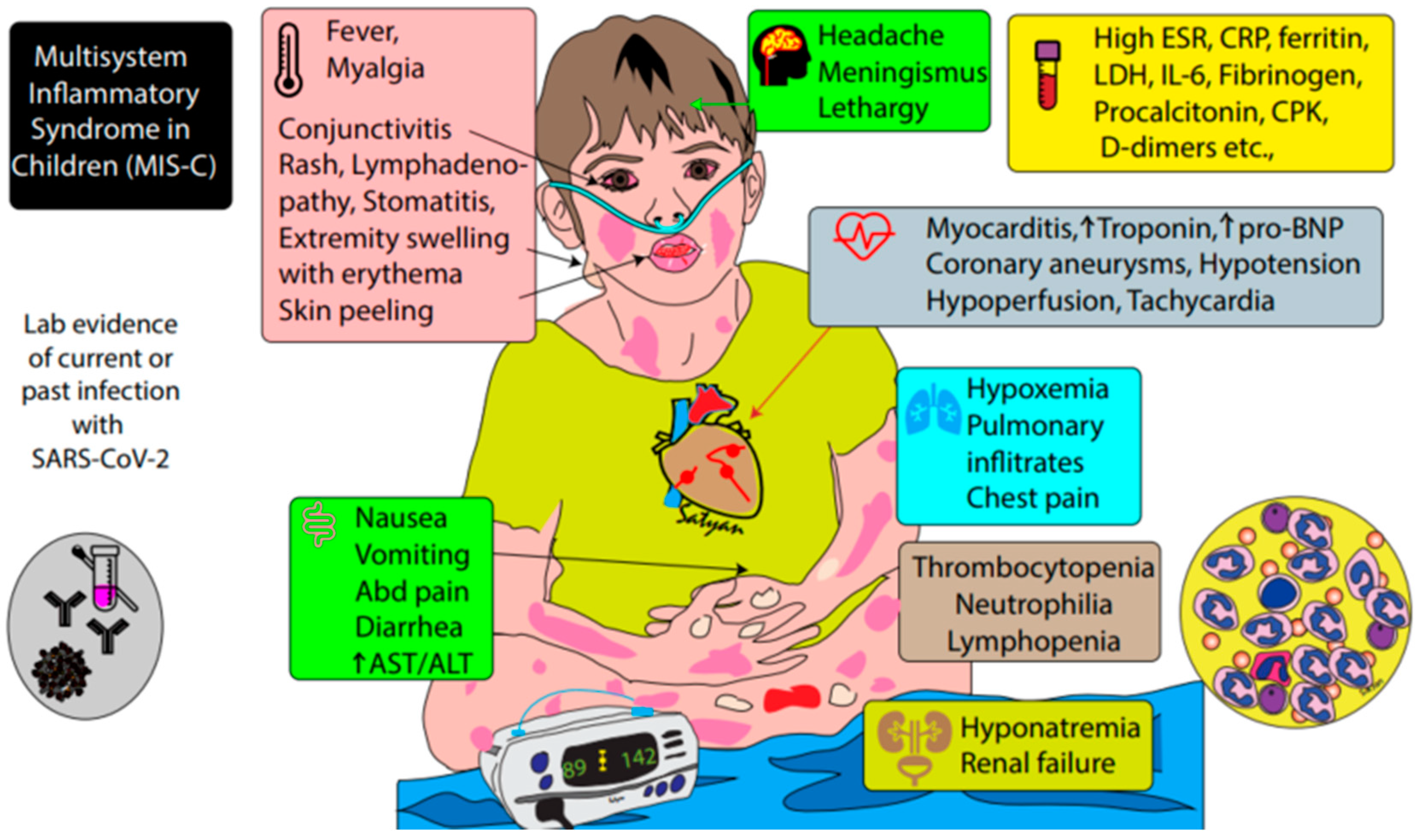
2. Definition of MIS-C
- (1)
- An individual aged < 21 years with:
- (2)
- Clinical criteria:
- A minimum 24-h history of subjective or objective fever ≥ 38.0 °C AND
- Severe illness necessitating hospitalization AND
- Two or more organ systems affected (i.e., cardiac, renal, respiratory, hematologic, gastrointestinal, dermatologic, neurological)
- (3)
- Laboratory evidence of inflammation
- One or more of the following: an elevated CRP, ESR, fibrinogen, procalcitonin, D-dimer, ferritin, LDH, or IL-6; elevated neutrophils or reduced lymphocytes; low albumin
- (4)
- Laboratory or epidemiologic evidence of SARS-CoV-2 infection
- Positive SARS-CoV-2 testing by RT-PCR, serology, or antigen OR
- COVID-19 exposure within 4 weeks prior to onset of symptoms
- (5)
- No alternative diagnosis
3. Clinical Presentation of MIS-C and Patient Outcomes
4. Relationship of MIS-C to COVID-19 and Pathogenesis
5. Comparison of MIS-C with Known Syndromes
5.1. Kawasaki Disease (KD)
5.2. Toxic Shock Syndrome (TSS)
5.3. Secondary Hemophagocytic Lymphohistiocytosis/Macrophage Activation Syndrome (SHLH/MAS)
6. Proposed Clinical Evaluation of Suspected MIS-C
- Consider observation in unit with cardio-respiratory monitoring capabilities
- Laboratory evaluation
- Complete blood count with differential
- Blood chemistry, including BUN and creatinine
- Liver function tests (ALT, AST, albumin, bilirubin)
- Cardiac markers: troponin and pro-BNP
- Urinalysis with culture if indicated
- Blood gas with lactate
- Markers of inflammation: ESR, CRP, procalcitonin, ferritin, triglycerides, IL-6 if available
- Coagulation panel: PT, PTT, fibrinogen, D-dimer
- Creatinine kinase, lactate dehydrogenase
- Blood culture
- Serology for SARS-CoV-2
- NP swab or lower respiratory tract sample for SARS-CoV-2 by RT-PCR; consider sending from stool if presenting with GI symptoms
- Additional studies as indicated: respiratory pathogen panel from NP swab or lower respiratory tract, stool studies/cultures, viral blood PCRs or serologies to rule out other causes of myocarditis, genetic testing for HLH, soluble IL-2 receptor, NK cell function
- Imaging:
- Chest X-ray
- Abdominal ultrasound or CT scan if concerning symptoms/physical findings
- Twelve-lead electrocardiogram (EKG)
- Echocardiogram (transthoracic)
- Early consultation of specialists to assist in management, such as intensive care, cardiology, rheumatology, infectious diseases, allergy/immunology, neurology
7. Suggested Management of Patients with MIS-C
7.1. Clinical Features Consistent with KD
7.2. Clinical Features Consistent with SHLH
7.3. Outpatient Management of Children under Investigation for MIS-C
7.4. Other Considerations
8. Future Directions
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- CDC COVID-19 Response Team. Coronavirus disease 2019 in children—United States, February 12–April 2, 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Parri, N.; Lenge, M.; Buonsenso, D. Children with Covid-19 in Pediatric Emergency Departments in Italy. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Riphagen, S.; Gomez, X.; Gonzalez-Martinez, C.; Wilkinson, N.; Theocharis, P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020. [Google Scholar] [CrossRef]
- Verdoni, L.; Mazza, A.; Gervasoni, A.; Martelli, L.; Ruggeri, M.; Ciuffreda, M.; Bonanomi, E.; D’Antiga, L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: An observational cohort study. Lancet 2020. [Google Scholar] [CrossRef]
- CDC Health Alert Network. Mulitsystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19). Available online: https://emergency.cdc.gov/han/2020/han00432.asp (accessed on 23 May 2020).
- Belhadjer, Z.; Meot, M.; Bajolle, F.; Khraiche, D.; Legendre, A.; Abakka, S.; Auriau, J.; Grimaud, M.; Oualha, M.; Beghetti, M.; et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation 2020. [Google Scholar] [CrossRef]
- Toubiana, J.P.C.; Corsia, A.; Bajolle, F.; Fourgeaud, J.; Angoulvant, F.; Debray, A.; Basmaci, R.; Salvador, E.; Biscardi, S.; Frange, P.; et al. Outbreak of Kawasaki Disease in Children during COVID-19 Pandemic: A Prospective Observational Study in Paris, France. Pre-Print. Available online: https://0-doi-org.brum.beds.ac.uk/10.1101/2020.05.10.20097394 (accessed on 25 May 2020).
- Belluck, P. New inflammatory condition in children probably linked to coronavirus, study finds. New York Times, 13 May 2020. [Google Scholar]
- Jones, V.G.; Mills, M.; Suarez, D.; Hogan, C.A.; Yeh, D.; Bradley Segal, J.; Nguyen, E.L.; Barsh, G.R.; Maskatia, S.; Mathew, R. COVID-19 and Kawasaki disease: Novel virus and novel case. Hosp. Pediatr. 2020. [Google Scholar] [CrossRef]
- Song, E.; Kajon, A.E.; Wang, H.; Salamon, D.; Texter, K.; Ramilo, O.; Leber, A.; Jaggi, P. Clinical and virologic characteristics may aid distinction of acute adenovirus disease from Kawasaki disease with incidental adenovirus detection. J. Pediatr. 2016, 170, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Health Advisory: Pediatric Multi-System Inflammatory Syndrome Temporally Associated with COVID-19 Interim Case Definition in New York State. Available online: https://health.ny.gov/press/releases/2020/docs/2020-05-13_health_advisory.pdf (accessed on 13 May 2020).
- Rawat, M.; Chandrasekharan, P.; Hicar, M.D.; Lakshminrusimha, S. COVID-19 in newborns and infants-low risk of severe disease: Silver lining or dark cloud? Am. J. Perinatol. 2020. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Do, A.; Vicencio, A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA 2020. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.E.; Katsaounou, P.; et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe 2020. [Google Scholar] [CrossRef]
- Fujimaru, T.; Ito, S.; Masuda, H.; Oana, S.; Kamei, K.; Ishiguro, A.; Kato, H.; Abe, J. Decreased levels of inflammatory cytokines in immunoglobulin-resistant Kawasaki disease after plasma exchange. Cytokine 2014, 70, 156–160. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A scientific statement for health professionals from the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef] [PubMed]
- Shulman, S.T. Pediatric COVID-associated Multi-system Inflammatory Syndrome (PMIS). J. Pediatr. Infect. Dis. Soc. 2020. [Google Scholar] [CrossRef]
- Rowley, A.H.; Shulman, S.T. The epidemiology and pathogenesis of Kawasaki disease. Front. Pediatr. 2018, 6, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esper, F.; Shapiro, E.D.; Weibel, C.; Ferguson, D.; Landry, M.L.; Kahn, J.S. Association between a novel human coronavirus and Kawasaki disease. J. Infect. Dis. 2005, 191, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.C.; Franco, A. The immunomodulatory effects of intravenous immunoglobulin therapy in Kawasaki disease. Expert Rev. Clin. Immunol. 2015, 11, 819–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanegaye, J.T.; Wilder, M.S.; Molkara, D.; Frazer, J.R.; Pancheri, J.; Tremoulet, A.H.; Watson, V.E.; Best, B.M.; Burns, J.C. Recognition of a Kawasaki disease shock syndrome. Pediatrics 2009, 123, e783–e789. [Google Scholar] [CrossRef] [Green Version]
- Gatterre, P.; Oualha, M.; Dupic, L.; Iserin, F.; Bodemer, C.; Lesage, F.; Hubert, P. Kawasaki disease: An unexpected etiology of shock and multiple organ dysfunction syndrome. Intensive Care Med. 2012, 38, 872–878. [Google Scholar] [CrossRef]
- Gamez-Gonzalez, L.B.; Moribe-Quintero, I.; Cisneros-Castolo, M.; Varela-Ortiz, J.; Munoz-Ramirez, M.; Garrido-Garcia, M.; Yamazaki-Nakashimada, M. Kawasaki disease shock syndrome: Unique and severe subtype of Kawasaki disease. Pediatr. Int. 2018, 60, 781–790. [Google Scholar] [CrossRef]
- Li, Y.; Luo, C.; Li, W.; Xu, Z.; Zeng, C.; Bi, S.; Yu, J.; Wu, J.; Yang, H. Structure-based preliminary analysis of immunity and virulence of SARS coronavirus. Viral Immunol. 2004, 17, 528–534. [Google Scholar] [CrossRef]
- Chuang, Y.Y.; Huang, Y.C.; Lin, T.Y. Toxic shock syndrome in children: Epidemiology, pathogenesis, and management. Paediatr. Drugs 2005, 7, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Parks, T.; Wilson, C.; Curtis, N.; Norrby-Teglund, A.; Sriskandan, S. Polyspecific intravenous immunoglobulin in clindamycin-treated patients with streptococcal toxic shock syndrome: A systematic review and meta-analysis. Clin. Infect. Dis. 2018, 67, 1434–1436. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.J.; Cheng, M.C.; Lo, M.H.; Chien, S.J. Early differentiation of Kawasaki disease shock syndrome and toxic shock syndrome in a pediatric intensive care unit. Pediatr. Infect. Dis. J. 2015, 34, 1163–1167. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.A.; Canna, S.W.; Schulert, G.S.; Volpi, S.; Lee, P.Y.; Kernan, K.F.; Caricchio, R.; Mahmud, S.; Hazen, M.M.; Halyabar, O.; et al. On the alert for cytokine storm: Immunopathology in COVID-19. Arthritis Rheumatol. 2020. [Google Scholar] [CrossRef] [Green Version]
- Grom, A.A.; Horne, A.; De Benedetti, F. Macrophage activation syndrome in the era of biologic therapy. Nat. Rev. Rheumatol. 2016, 12, 259–268. [Google Scholar] [CrossRef]
- Simon, D.W.; Halstead, E.S.; Davila, S.; Kernan, K.F.; Clark, R.S.B.; Storch, G.; Carcillo, J.A. DNA viremia is associated with hyperferritinemia in pediatric sepsis. J. Pediatr. 2019, 213, 82–87. [Google Scholar] [CrossRef]
- Chesshyre, E.; Ramanan, A.V.; Roderick, M.R. Hemophagocytic lymphohistiocytosis and infections: An update. Pediatr. Infect. Dis. J. 2019, 38, e54–e56. [Google Scholar] [CrossRef]
- Latino, G.A.; Manlhiot, C.; Yeung, R.S.; Chahal, N.; McCrindle, B.W. Macrophage activation syndrome in the acute phase of Kawasaki disease. J. Pediatr. Hematol. Oncol. 2010, 32, 527–531. [Google Scholar] [CrossRef]
- Vastert, S.J.; van Wijk, R.; D’Urbano, L.E.; de Vooght, K.M.; de Jager, W.; Ravelli, A.; Magni-Manzoni, S.; Insalaco, A.; Cortis, E.; van Solinge, W.W.; et al. Mutations in the perforin gene can be linked to macrophage activation syndrome in patients with systemic onset juvenile idiopathic arthritis. Rheumatolology 2010, 49, 441–449. [Google Scholar] [CrossRef] [Green Version]
- Halyabar, O.; Chang, M.H.; Schoettler, M.L.; Schwartz, M.A.; Baris, E.H.; Benson, L.A.; Biggs, C.M.; Gorman, M.; Lehmann, L.; Lo, M.S.; et al. Calm in the midst of cytokine storm: A collaborative approach to the diagnosis and treatment of hemophagocytic lymphohistiocytosis and macrophage activation syndrome. Pediatr. Rheumatol. Online J. 2019, 17, 7. [Google Scholar] [CrossRef] [Green Version]
- Schram, A.M.; Campigotto, F.; Mullally, A.; Fogerty, A.; Massarotti, E.; Neuberg, D.; Berliner, N. Marked hyperferritinemia does not predict for HLH in the adult population. Blood 2015, 125, 1548–1552. [Google Scholar] [CrossRef] [PubMed]
- Henter, J.I.; Horne, A.; Arico, M.; Egeler, R.M.; Filipovich, A.H.; Imashuku, S.; Ladisch, S.; McClain, K.; Webb, D.; Winiarski, J.; et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr. Blood Cancer 2007, 48, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, A.; Minoia, F.; Davi, S.; Horne, A.; Bovis, F.; Pistorio, A.; Arico, M.; Avcin, T.; Behrens, E.M.; De Benedetti, F.; et al. 2016 classification criteria for macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: A European league against rheumatism/American college of rheumatology/paediatric rheumatology international trials organisation collaborative initiative. Ann. Rheum. Dis. 2016, 75, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Davi, S.; Minoia, F.; Pistorio, A.; Horne, A.; Consolaro, A.; Rosina, S.; Bovis, F.; Cimaz, R.; Gamir, M.L.; Ilowite, N.T.; et al. Performance of current guidelines for diagnosis of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Arthritis Rheumatol. 2014, 66, 2871–2880. [Google Scholar] [CrossRef]
- de Graeff, N.; Groot, N.; Ozen, S.; Eleftheriou, D.; Avcin, T.; Bader-Meunier, B.; Dolezalova, P.; Feldman, B.M.; Kone-Paut, I.; Lahdenne, P.; et al. European consensus-based recommendations for the diagnosis and treatment of Kawasaki disease—The SHARE initiative. Rheumatology 2019, 58, 672–682. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. Available online: https://clinicaltrials.gov/ct2/results?cond=covid-19+tocilizumab&term=&cntry=&state=&city=&dist=&Search=Search (accessed on 20 May 2020).
- Kobayashi, T.; Inoue, Y.; Takeuchi, K.; Okada, Y.; Tamura, K.; Tomomasa, T.; Kobayashi, T.; Morikawa, A. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation 2006, 113, 2606–2612. [Google Scholar] [CrossRef]
- Chen, S.; Dong, Y.; Yin, Y.; Krucoff, M.W. Intravenous immunoglobulin plus corticosteroid to prevent coronary artery abnormalities in Kawasaki disease: A meta-analysis. Heart 2013, 99, 76–82. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Saji, T.; Otani, T.; Takeuchi, K.; Nakamura, T.; Arakawa, H.; Kato, T.; Hara, T.; Hamaoka, K.; Ogawa, S.; et al. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): A randomised, open-label, blinded-endpoints trial. Lancet 2012, 379, 1613–1620. [Google Scholar] [CrossRef]
- Guillaume, M.P.; Reumaux, H.; Dubos, F. Usefulness and safety of anakinra in refractory Kawasaki disease complicated by coronary artery aneurysm. Cardiol. Young 2018, 28, 739–742. [Google Scholar] [CrossRef]
- Nozawa, T.; Imagawa, T.; Ito, S. Coronary-artery aneurysm in tocilizumab-treated children with Kawasaki’s disease. N. Engl. J. Med. 2017, 377, 1894–1896. [Google Scholar] [CrossRef]
- Evans, J.; Steel, L.; Borg, F.; Dasgupta, B. Long-term efficacy and safety of tocilizumab in giant cell arteritis and large vessel vasculitis. RMD Open 2016, 2, e000137. [Google Scholar] [CrossRef] [Green Version]
- Antinori, S.; Bonazzetti, C.; Gubertini, G.; Capetti, A.; Pagani, C.; Morena, V.; Rimoldi, S.; Galimberti, L.; Sarzi-Puttini, P.; Ridolfo, A.L. Tocilizumab for cytokine storm syndrome in COVID-19 pneumonia: An increased risk for candidemia? Autoimmun. Rev. 2020. [Google Scholar] [CrossRef]
- Shakoory, B.; Carcillo, J.A.; Chatham, W.W.; Amdur, R.L.; Zhao, H.; Dinarello, C.A.; Cron, R.Q.; Opal, S.M. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: Reanalysis of a prior phase III trial. Crit. Care Med. 2016, 44, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- Beverley, P.C. Immunology of vaccination. Br. Med. Bull. 2002, 62, 15–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jares Baglivo, S.; Polack, F.P. The long road to protect infants against severe RSV lower respiratory tract illness. F1000 Res. 2019, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef] [Green Version]
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Foley, B.; Giorgi, E.E.; Bhattacharya, T.; Parker, M.D.; et al. Spike Mutation Pipeline Reveals the Emergence of a More Transmissible Form of SARS-CoV-2. 2020. Available online: https://0-doi-org.brum.beds.ac.uk/10.1101/2020.04.29.069054 (accessed on 26 May 2020).
- Swaminathan, S.; Khanna, N. Dengue vaccine development: Global and Indian scenarios. Int. J. Infect. Dis. 2019, 84S, S80–S86. [Google Scholar] [CrossRef] [Green Version]

| Pediatric MIS-C | Kawasaki Disease (KD) | Kawasaki Disease Shock Syndrome (KDSS) | Toxic Shock Syndrome (TSS) | |
|---|---|---|---|---|
| Age of affected children | Older (range 6 m–16 y) | Younger | Younger | Older |
| Hypotension | ± | − | ++ | ++ |
| Mucous membrane involvement | ± | + | + | ± |
| Rash | + | + | + | Typically erythroderma |
| Desquamation | + | + | + | + |
| Altered mental status or encephalopathy | + | Rare | + | + |
| Vomiting, diarrhea, and/or abdominal pain | ++ | Rare | + | + |
| Respiratory distress | + | Rare | + | ± |
| Myalgias | + | − | − | + |
| WBC differential | Neutrophilia, lymphopenia | Neutrophilia | Neutrophilia | Neutrophilia |
| Platelets | ↓ | ↑ | ↓, normal, or ↑ | ↓ |
| PT/PTT | ↑ | normal | normal or ↑ | ↑ |
| Fibrinogen | ↓, normal, or ↑ | normal | normal, or ↑ | ↓ |
| D-dimer | ↑ | normal | normal, or ↑ | ↑ |
| ALT | normal, or ↑ | normal, or ↑ | normal, or ↑ | normal, or ↑ |
| Creatinine | ↑ | normal | ↑ | ↑ |
| Troponin | ↑ | normal, or ↑ | ↑ | ID |
| Pro-BNP | ⇈ | normal, or ↑ | ↑ | ID |
| Ferritin | ↑ | normal, or ↑ | normal, or ↑ | normal |
| CRP | ⇈ | ↑ | ⇈ | ↑ |
| Coronary artery dilation or aneurysms | + | + | ++ | − |
| Cardiac ventricular dysfunction | + | ± | + | Rare |
| Valvular regurgitation | + | + | ++ | Rare |
| HLH Diagnosis Can Be Established with either 1 or 2. |
|---|
|
| Medication Class | Dose | Important Notes |
|---|---|---|
| IVIG [16,34] |
| Use with caution if fluid overload, renal dysfunction. Consider alternate dosing strategy. |
| Aspirin |
| Precaution in severe thrombocytopenia |
| Corticosteroids [34,39] | For severe KD *:
| Precaution if positive RT-PCR for SARS-CoV-2, suggesting active infection |
| Anakinra [16,34] |
| |
| Tocilizumab |
| Trials ongoing for safety and efficacy in the setting of active coronavirus infection [40] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakra, N.A.; Blumberg, D.A.; Herrera-Guerra, A.; Lakshminrusimha, S. Multi-System Inflammatory Syndrome in Children (MIS-C) Following SARS-CoV-2 Infection: Review of Clinical Presentation, Hypothetical Pathogenesis, and Proposed Management. Children 2020, 7, 69. https://0-doi-org.brum.beds.ac.uk/10.3390/children7070069
Nakra NA, Blumberg DA, Herrera-Guerra A, Lakshminrusimha S. Multi-System Inflammatory Syndrome in Children (MIS-C) Following SARS-CoV-2 Infection: Review of Clinical Presentation, Hypothetical Pathogenesis, and Proposed Management. Children. 2020; 7(7):69. https://0-doi-org.brum.beds.ac.uk/10.3390/children7070069
Chicago/Turabian StyleNakra, Natasha A., Dean A. Blumberg, Angel Herrera-Guerra, and Satyan Lakshminrusimha. 2020. "Multi-System Inflammatory Syndrome in Children (MIS-C) Following SARS-CoV-2 Infection: Review of Clinical Presentation, Hypothetical Pathogenesis, and Proposed Management" Children 7, no. 7: 69. https://0-doi-org.brum.beds.ac.uk/10.3390/children7070069





