AQP1-Driven Migration Is Independent of Other Known Adverse Factors but Requires a Hypoxic Undifferentiated Cell Profile in Neuroblastoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Tumor Tissue
2.3. In Vitro Wound Healing
2.4. Modified Migration Assay
2.5. RNA Isolation/cDNA Synthesis/qPCR
2.6. Immunofluorescence Staining
2.7. Flow Cytometry
2.8. Statistical Analysis
3. Results
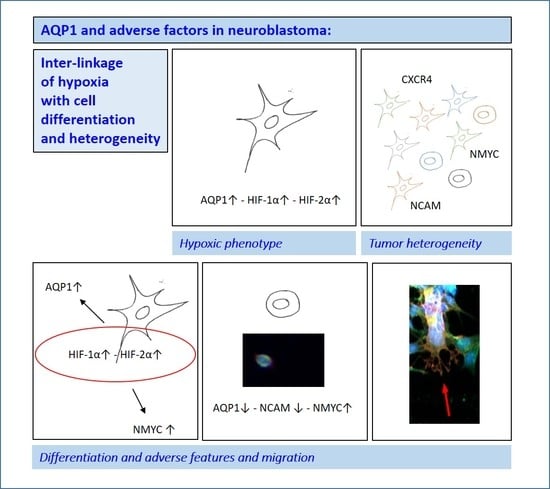
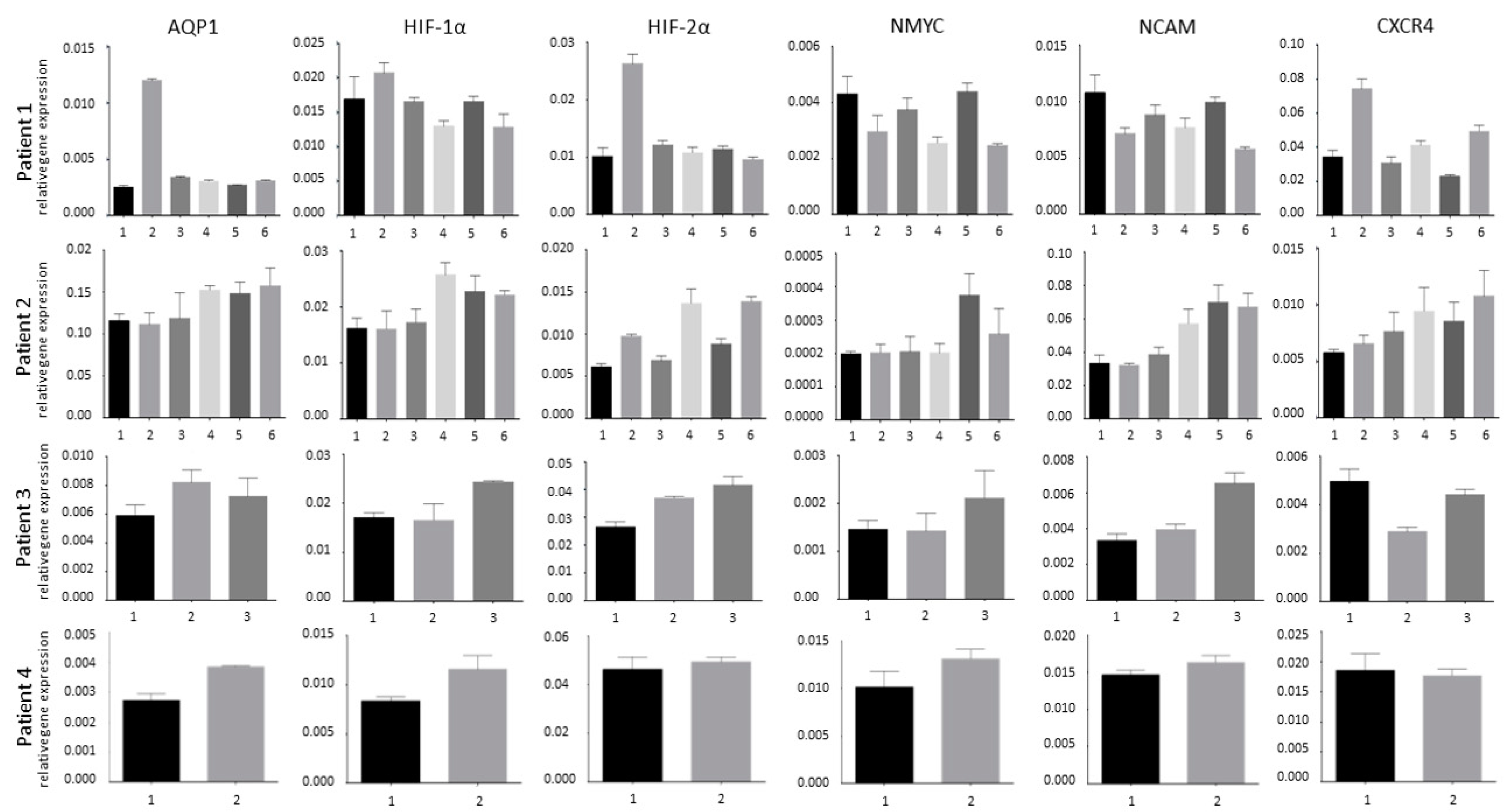
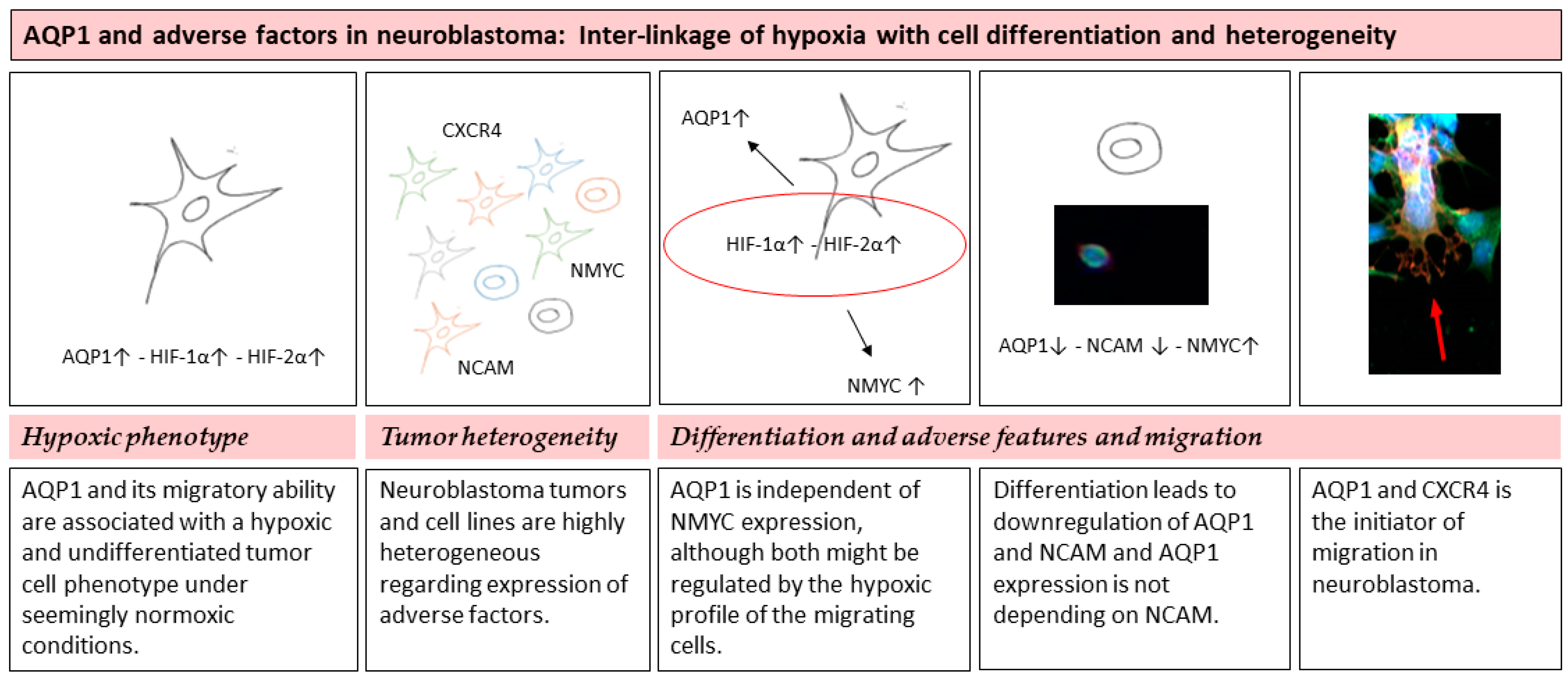
3.1. Tumor Heterogeneity
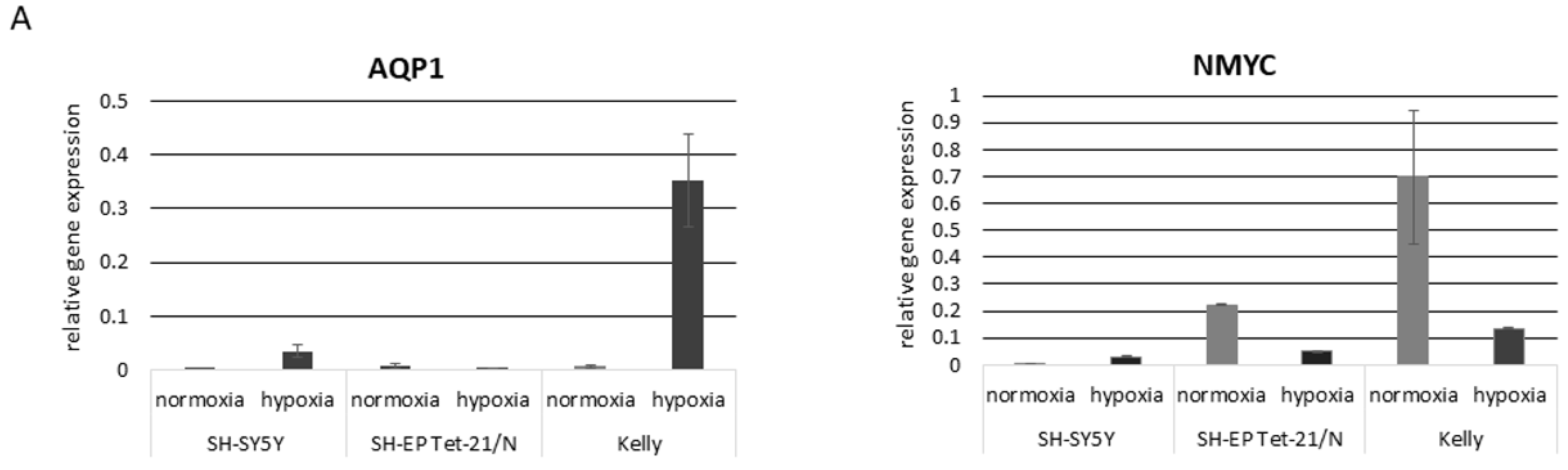
3.2. Hypoxia and Unfavorable Properties
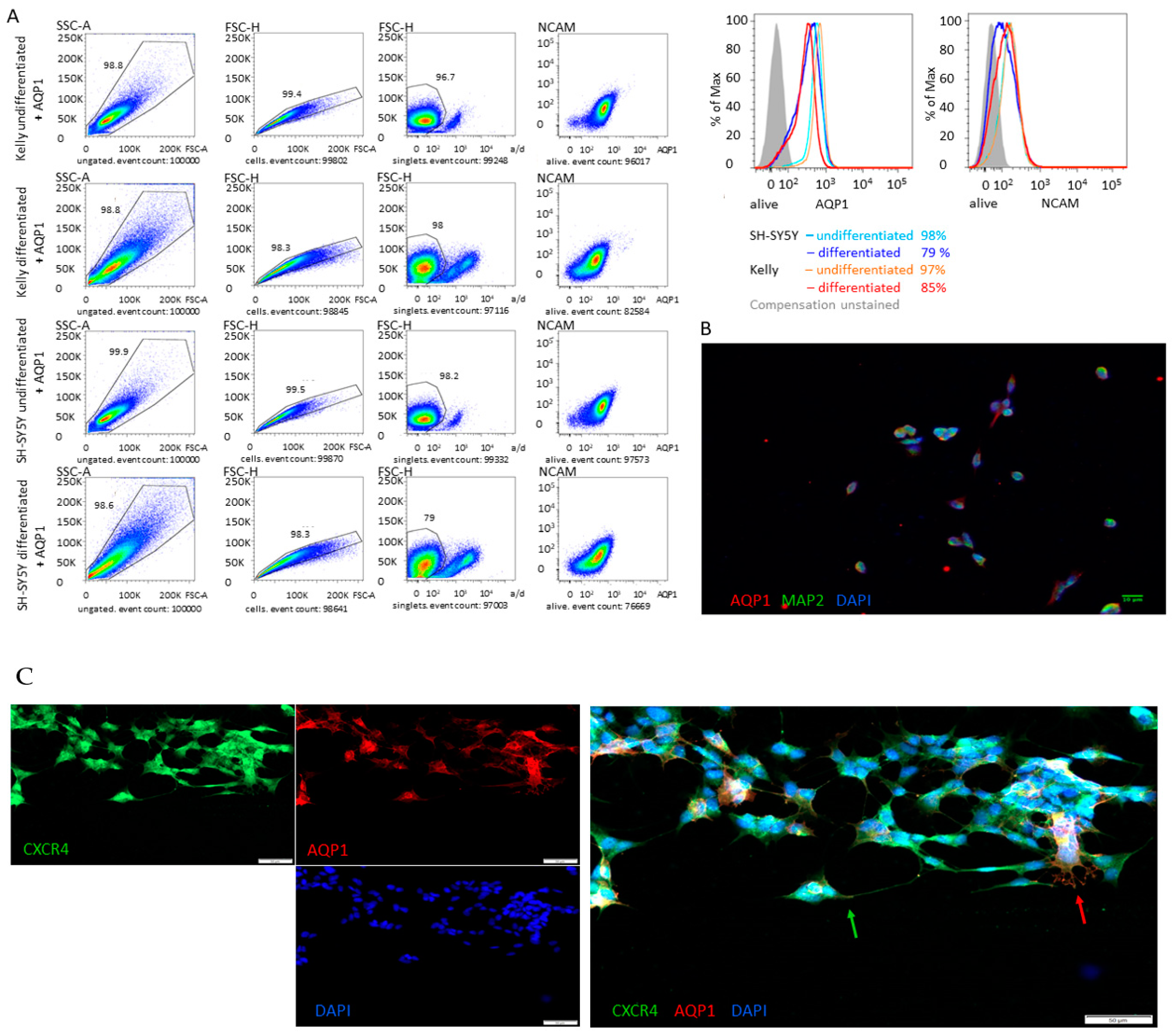
3.3. Differentiation
3.4. Migration is Independent of CXCR4
4. Discussion
4.1. Tumor Heterogeneity
4.2. Hypoxic Phenotype
4.3. Differentiation and Adverse Features and Migration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boeva, V.; Louis-Brennetot, C.; Peltier, A.; Durand, S.; Pierre-Eugène, C.; Raynal, V.; Etchevers, H.C.; Thomas, S.; Lermine, A.; Daudigeos-Dubus, E.; et al. Heterogeneity of neuroblastoma cell identity defined by transcriptional circuitries. Nat. Genet. 2017, 49, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Ohira, M.; Morohashi, A.; Inuzuka, H.; Shishikura, T.; Kawamoto, T.; Kageyama, H.; Nakamura, Y.; Isogai, E.; Takayasu, H.; Sakiyama, S.; et al. Expression profiling and characterization of 4200 genes cloned from primary neuroblastomas: Identification of 305 genes differentially expressed between favorable and unfavorable subsets. Oncogene 2003, 22, 5525–5536. [Google Scholar] [CrossRef] [Green Version]
- Shipley, M.M.; Mangold, C.A.; Szpara, M.L. Differentiation of the SH-SY5Y Human Neuroblastoma Cell Line. J. Vis. Exp. 2016, 2016, e53193. [Google Scholar] [CrossRef] [PubMed]
- Bosse, K.R.; Maris, J.M. Advances in the translational genomics of neuroblastoma: From improving risk stratification and revealing novel biology to identifying actionable genomic alterations. Cancer 2016, 122, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Westermark, U.K.; Wilhelm, M.; Frenzel, A.; Henriksson, M.A. The MYCN oncogene and differentiation in neuroblastoma. Semin. Cancer Biol. 2011, 21, 256–266. [Google Scholar] [CrossRef]
- Wakamatsu, Y.; Watanabe, Y.; Nakamura, H.; Kondoh, H. Regulation of the neural crest cell fate by N-myc: Promotion of ventral migration and neuronal differentiation. Development 1997, 124, 1953–1962. [Google Scholar]
- Kang, J.-H.; Rychahou, P.G.; Ishola, T.A.; Qiao, J.; Evers, B.M.; Chung, D.H. MYCN silencing induces differentiation and apoptosis in human neuroblastoma cells. Biochem. Biophys. Res. Commun. 2006, 351, 192–197. [Google Scholar] [CrossRef] [Green Version]
- Aygun, N. Biological and Genetic Features of Neuroblastoma and Their Clinical Importance. Curr. Pediatr. Rev. 2018, 14, 73–90. [Google Scholar] [CrossRef]
- Zecchini, S.; Bombardelli, L.; Decio, A.; Bianchi, M.; Mazzarol, G.; Sanguineti, F.; Aletti, G.D.; Maddaluno, L.; Berezin, V.; Bock, E.; et al. The adhesion molecule NCAM promotes ovarian cancer progression via FGFR signalling. EMBO Mol. Med. 2011, 3, 480–494. [Google Scholar] [CrossRef]
- Markovsky, E.; Eldar-Boock, A.; Ben-Shushan, D.; Baabur-Cohen, H.; Yeini, E.; Pisarevsky, E.; Many, A.; Aviel-Ronen, S.; Barshack, I.; Satchi-Fainaro, R. Targeting NCAM-expressing neuroblastoma with polymeric precision nanomedicine. J. Control. Release 2017, 249, 162–172. [Google Scholar] [CrossRef]
- Ferlemann, F.C.; Menon, V.; Condurat, A.L.; Rößler, J.; Pruszak, J. Surface marker profiling of SH-SY5Y cells enables small molecule screens identifying BMP4 as a modulator of neuroblastoma differentiation. Sci. Rep. 2017, 7, 13612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaifi, J.T.; Yekebas, E.F.; Schurr, P.; Obonyo, D.; Wachowiak, R.; Busch, P.; Heinecke, A.; Pantel, K.; Izbicki, J.R. Tumor-Cell Homing to Lymph Nodes and Bone Marrow and CXCR4 Expression in Esophageal Cancer. J. Natl. Cancer Inst. 2005, 97, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Russell, H.V.; Hicks, J.; Okcu, M.F.; Nuchtern, J.G. CXCR4 Expression in neuroblastoma primary tumors is associated with clinical presentation of bone and bone marrow metastases. J. Pediatr. Surg. 2004, 39, 1506–1511. [Google Scholar] [CrossRef] [PubMed]
- Ameis, H.M.; Drenckhan, A.; Von Loga, K.; Escherich, G.; Wenke, K.; Izbicki, J.R.; Reinshagen, K.; Gros, S.J. PGK1 as Predictor of CXCR4 Expression, Bone Marrow Metastases and Survival in Neuroblastoma. PLoS ONE 2013, 8, e83701. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Hara-Chikuma, M.; Papadopoulos, M.C. Papadopoulos, Aquaporins—new players in cancer biology. J. Mol. Med. 2008, 86, 523–529. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, M.C.; Saadoun, S.; Verkman, A.S. Aquaporins and cell migration. Pflügers Archiv. Eur. J. Physiol. 2007, 456, 693–700. [Google Scholar] [CrossRef] [Green Version]
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of Water Channels in Xenopus Oocytes Expressing Red Cell CHIP28 Protein. Science 1992, 256, 385–387. [Google Scholar] [CrossRef] [Green Version]
- Saadoun, S.; Papadopoulos, M.C.; Hara-Chikuma, M.; Verkman, A.S. Impairment of angiogenesis and cell migration by targeted aquaporin-1 gene disruption. Nat. Cell Biol. 2005, 434, 786–792. [Google Scholar] [CrossRef]
- Bhaskara, V.K.; Mohanam, I.; Rao, J.S.; Mohanam, S. Intermittent Hypoxia Regulates Stem-like Characteristics and Differentiation of Neuroblastoma Cells. PLoS ONE 2012, 7, e30905. [Google Scholar] [CrossRef] [Green Version]
- Cimmino, F.; Pezone, L.; Avitabile, M.; Acierno, G.; Andolfo, I.; Capasso, M.; Iolascon, A. Inhibition of hypoxia inducible factors combined with all-trans retinoic acid treatment enhances glial transdifferentiation of neuroblastoma cells. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Westerlund, I.; Shi, Y.; Toskas, K.; Fell, S.M.; Li, S.; Surova, O.; Södersten, E.; Kogner, P.; Nyman, U.; Schlisio, S.; et al. Combined epigenetic and differentiation-based treatment inhibits neuroblastoma tumor growth and links HIF2α to tumor suppression. Proc. Natl. Acad. Sci. USA 2017, 114, E6137–E6146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmquist, L.; Jögi, A.; Påhlman, S. Phenotypic persistence after reoxygenation of hypoxic neuroblastoma cells. Int. J. Cancer 2005, 116, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Jögi, A.; Øra, I.; Nilsson, H.; Lindeheim, Å.; Makino, Y.; Poellinger, L.; Axelson, H.; Påhlman, S. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc. Natl. Acad. Sci. USA 2002, 99, 7021–7026. [Google Scholar]
- Jögi, A.; Vallon-Christersson, J.; Holmquist, L.; Axelson, H.; Borg, A.; Påhlman, S. Human neuroblastoma cells exposed to hypoxia: Induction of genes associated with growth, survival, and aggressive behavior. Exp. Cell Res. 2004, 295, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Lutz, W.; Fulda, S.; Jeremias, I.; Debatin, K.; Schwab, M. MycN and IFNgamma cooperate in apoptosis of human neuroblastoma cells. Oncogene 1998, 17, 339–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutz, W.; Stöhr, M.; Schürmann, J.; Wenzel, A.; Löhr, A.; Schwab, M. Conditional expression of N-myc in human neuroblastoma cells increases expression of alpha-prothymosin and ornithine decarboxylase and accelerates progression into S-phase early after mitogenic stimulation of quiescent cells. Oncogene 1996, 13, 803–812. [Google Scholar]
- Lee, H.; Goetzl, E.J.; An, S. Lysophosphatidic acid and sphingosine 1-phosphate stimulate endothelial cell wound healing. Am. J. Physiol. Physiol. 2000, 278, C612–C618. [Google Scholar] [CrossRef]
- Carnahan, J.F.; Patterson, P.H. Isolation of the progenitor cells of the sympathoadrenal lineage from embryonic sympathetic ganglia with the SA monoclonal antibodies. J. Neurosci. 1991, 11, 3520–3530. [Google Scholar] [CrossRef] [Green Version]
- Verdi, J.M.; Anderson, D.J. Neurotrophins regulate sequential changes in neurotrophin receptor expression by sympathetic neuroblasts. Neuron 1994, 13, 1359–1372. [Google Scholar] [CrossRef]
- Axelson, H. The Notch signaling cascade in neuroblastoma: Role of the basic helix-loop-helix proteins HASH-1 and HES-1. Cancer Lett. 2004, 204, 171–178. [Google Scholar] [CrossRef]
- Semenza, G.L. Hypoxia, Clonal Selection, and the Role of HIF-1 in Tumor Progression. Crit. Rev. Biochem. Mol. Biol. 2000, 35, 71–103. [Google Scholar] [CrossRef] [PubMed]
- Brizel, D.M.; Scully, S.P.; Harrelson, J.M.; Layfield, L.J.; Bean, J.M.; Prosnitz, L.R.; Dewhirst, M.W. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer Res. 1996, 56, 941–943. [Google Scholar] [PubMed]
- Metzen, E.; Wolff, M.; Fandrey, J.; Jelkmann, W. Pericellular PO2 and O2 consumption in monolayer cell cultures. Respir. Physiol. 1995, 100, 101–106. [Google Scholar] [CrossRef]
- Nilsson, H.; Jögi, A.; Beckman, S.; Harris, A.L.; Poellinger, L.; Påhlman, S. HIF-2alpha expression in human fetal paraganglia and neuroblastoma: Relation to sympathetic differentiation, glucose deficiency, and hypoxia. Exp. Cell Res. 2005, 303, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Fredlund, E.; Ovenberger, M.; Borg, A.; Påhlman, S. Transcriptional adaptation of neuroblastoma cells to hypoxia. Biochem. Biophys. Res. Commun. 2008, 366, 1054–1060. [Google Scholar] [CrossRef]
- Qing, G.; Skuli, N.; Mayes, P.A.; Pawel, B.; Martinez, D.; Maris, J.M.; Simon, M.C. Combinatorial Regulation of Neuroblastoma Tumor Progression by N-Myc and Hypoxia Inducible Factor HIF-1α. Cancer Res. 2010, 70, 10351–10361. [Google Scholar] [CrossRef] [Green Version]
- Zwaans, B.M.M.; Lombard, D.B. Interplay between sirtuins, MYC and hypoxia-inducible factor in cancer-associated metabolic reprogramming. Dis. Model. Mech. 2014, 7, 1023–1032. [Google Scholar] [CrossRef] [Green Version]
- Laemmle, A.; Lechleiter, A.; Roh, V.; Schwarz, C.; Portmann, S.; Furer, C.; Keogh, A.; Tschan, M.P.; Candinas, D.; Vorburger, S.A.; et al. Inhibition of SIRT1 Impairs the Accumulation and Transcriptional Activity of HIF-1α Protein under Hypoxic Conditions. PLoS ONE 2012, 7, e33433. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Sun, X.-X.; Qian, D.Z.; Dai, M. Molecular Crosstalk Between MYC and HIF in Cancer. Front. Cell Dev. Biol. 2020, 8. [Google Scholar] [CrossRef]
- Lim, J.-H.; Lee, Y.-M.; Chun, Y.-S.; Chen, J.; Kim, J.-E.; Park, J.-W. Sirtuin 1 Modulates Cellular Responses to Hypoxia by Deacetylating Hypoxia-Inducible Factor 1α. Mol. Cell 2010, 38, 864–878. [Google Scholar] [CrossRef]
- Chen, R.; Dioum, E.M.; Hogg, R.T.; Gerard, R.D.; Garcia, J.A. Hypoxia Increases Sirtuin 1 Expression in a Hypoxia-inducible Factor-dependent Manner. J. Biol. Chem. 2011, 286, 13869–13878. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurlin, P.J. N-myc functions in transcription and development. Birth Defects Res. Part C Emb. Today Rev. 2005, 75, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Moens, C.B.; Stanton, B.R.; Parada, L.F.; Rossant, J. Defects in heart and lung development in compound heterozygotes for two different targeted mutations at the N-myc locus. Development 1993, 119, 485–499. [Google Scholar] [PubMed]
- Stanton, B.R.; Perkins, A.S.; Tessarollo, L.A.; Sassoon, D.; Parada, L.F. Loss of N-myc function results in embryonic lethality and failure of the epithelial component of the embryo to develop. Genes Dev. 1992, 6, 2235–2247. [Google Scholar] [CrossRef] [PubMed]
- Moens, C.B.; Auerbach, A.B.A.; Conlon, R.; Joyner, A.L.; Rossant, J. A targeted mutation reveals a role for N-myc in branching morphogenesis in the embryonic mouse lung. Genes Dev. 1992, 6, 691–704. [Google Scholar] [CrossRef] [Green Version]
- Sawai, S.; Shimono, A.; Wakamatsu, Y.; Palmes, C.; Hanaoka, K.; Kondoh, H. Defects of embryonic organogenesis resulting from targeted disruption of the N-myc gene in the mouse. Development 1993, 117, 1445–1455. [Google Scholar]
- Charron, J.A.; Malynn, B.; Fisher, P.; Stewart, V.; Jeannotte, L.; Goff, S.P.; Robertson, E.J.; Alt, F.W. Embryonic lethality in mice homozygous for a targeted disruption of the N-myc gene. Genes Dev. 1992, 6, 2248–2257. [Google Scholar] [CrossRef]
- Thiele, C.J.; Reynolds, C.P.; Israel, M.A. Decreased expression of N-myc precedes retinoic acid-induced morphological differentiation of human neuroblastoma. Nat. Cell Biol. 1985, 313, 404–406. [Google Scholar] [CrossRef]
- Abemayor, E.; Sidell, N. Human neuroblastoma cell lines as models for the in vitro study of neoplastic and neuronal cell differentiation. Environ. Health Perspect. 1989, 80, 3–15. [Google Scholar] [CrossRef]
- Amatruda, T.T., III; Sidell, N.; Ranyard, J.; Koeffler, H.P. Retinoic acid treatment of human neuroblastoma cells is associated with decreased N-myc expression. Biochem. Biophys. Res. Commun. 1985, 126, 1189–1195. [Google Scholar] [CrossRef]
- Wada, R.K.; Seeger, R.C.; Reynolds, C.P.; Alloggiamento, T.; Yamashiro, J.M.; Ruland, C.; Black, A.C.; Rosenblatt, J.D. Cell type-specific expression and negative regulation by retinoic acid of the human N-myc promoter in neuroblastoma cells. Oncogene 1992, 7, 711–717. [Google Scholar] [PubMed]
- Abemayor, E.; Chang, B.; Sidell, N. Effects of retinoic acid on the in vivo growth of human neuroblastoma cells. Cancer Lett. 1990, 55, 1–5. [Google Scholar] [CrossRef]
- Matthay, K.K.; Reynolds, C.P.; Seeger, R.C.; Shimada, H.; Adkins, E.S.; Haas-Kogan, D.; Gerbing, R.B.; London, W.B.; Villablanca, J.G. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: A children’s oncology group study. J. Clin. Oncol. 2009, 27, 1007–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negroni, A.; Scarpa, S.; Romeo, A.; Ferrari, S.; Modesti, A.; Raschellà, G. Decrease of proliferation rate and induction of differentiation by a MYCN antisense DNA oligomer in a human neuroblastoma cell line. Cell Growth Differ. Mol. Boil. J. Am. Assoc. Cancer Res. 1991, 2, 511–518. [Google Scholar]
- Buechner, J.; Flaegstad, T.; Einvik, C.; Henriksen, J.R.; Haug, B.H.; Tømte, E.; Løkke, C. Conditional expression of retrovirally delivered anti-MYCN shRNA as an in vitro model system to study neuronal differentiation in MYCN-amplified neuroblastoma. BMC Dev. Biol. 2011, 11. [Google Scholar] [CrossRef] [Green Version]
- Buechner, J.; Henriksen, J.R.; Haug, B.H.; Tømte, E.; Flaegstad, T.; Einvik, C. Inhibition of mir-21, which is up-regulated during MYCN knockdown-mediated differentiation, does not prevent differentiation of neuroblastoma cells. Differentiation 2011, 81, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Brodeur, G.M.; Seeger, R.C.; Schwab, M.E.; Varmus, H.; Bishop, J.M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 1984, 224, 1121–1124. [Google Scholar] [CrossRef]
- Schwab, M.; Alitalo, K.; Klempnauer, K.-H.; Varmus, H.E.; Bishop, J.M.; Gilbert, F.; Brodeur, G.; Goldstein, M.; Trent, J. Amplified DNA with limited homology to myc cellular oncogene is shared by human neuroblastoma cell lines and a neuroblastoma tumour. Nat. Cell Biol. 1983, 305, 245–248. [Google Scholar] [CrossRef]
- Seeger, R.C.; Brodeur, G.M.; Sather, H.; Dalton, A.; Siegel, S.E.; Wong, K.Y.; Hammond, D. Association of Multiple Copies of the N-mycOncogene with Rapid Progression of Neuroblastomas. N. Engl. J. Med. 1985, 313, 1111–1116. [Google Scholar] [CrossRef]
- Powers, J.T.; Tsanov, K.M.; Pearson, D.S.; Roels, F.; Spina, C.S.; Ebright, R.; Seligson, M.; De Soysa, Y.; Cahan, P.; Theißen, J.; et al. Multiple mechanisms disrupt the let-7 microRNA family in neuroblastoma. Nat. Cell Biol. 2016, 535, 246–251. [Google Scholar] [CrossRef] [Green Version]
- Winter, C.; Pawel, B.; Seiser, E.; Zhao, H.; Raabe, E.H.; Wang, Q.; Judkins, A.R.; Attiyeh, E.; Maris, J.M. Neural cell adhesion molecule (NCAM) isoform expression is associated with neuroblastoma differentiation status. Pediatr. Blood Cancer 2008, 51, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Wachowiak, R.; Rawnaq, T.; Metzger, R.; Quaas, A.; Fiegel, H.; Kähler, N.; Rolle, U.; Izbicki, J.R.; Kaifi, J.; Till, H. Universal expression of cell adhesion molecule NCAM in neuroblastoma in contrast to L1: Implications for different roles in tumor biology of neuroblastoma? Pediatr. Surg. Int. 2008, 24, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, M.; Hirsch, M.-R.; Deagostini-Bazin, H.; Yamada, O.; Tursz, T.; Goridis, C. Characterization of neural cell adhesion molecules (NCAM) expressed by ewing and neuroblastoma cell lines. Int. J. Cancer 1987, 40, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Seifert, A.; Glanz, D.; Glaubitz, N.; Horstkorte, R.; Bork, K. Polysialylation of the neural cell adhesion molecule: Interfering with polysialylation and migration in neuroblastoma cells. Arch. Biochem. Biophys. 2012, 524, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Falconer, R.A.; Errington, R.J.; Shnyder, S.D.; Smith, P.J.; Patterson, L.H. Polysialyltransferase: A new target in metastatic cancer. Curr. Cancer Drug Targets 2012, 12, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Elkashef, S.M.; Allison, S.J.; Sadiq, M.; Basheer, H.A.; Morais, G.R.; Loadman, P.M.; Pors, K.; Falconer, R.A. Polysialic acid sustains cancer cell survival and migratory capacity in a hypoxic environment. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Daly, E.B.; Wind, T.; Jiang, X.-M.; Sun, L.; Hogg, P.J. Secretion of phosphoglycerate kinase from tumour cells is controlled by oxygen-sensing hydroxylases. Biochim. Biophys. Acta Bioenergy 2004, 1691, 17–22. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pini, N.; Huo, Z.; Kym, U.; Holland-Cunz, S.; Gros, S.J. AQP1-Driven Migration Is Independent of Other Known Adverse Factors but Requires a Hypoxic Undifferentiated Cell Profile in Neuroblastoma. Children 2021, 8, 48. https://0-doi-org.brum.beds.ac.uk/10.3390/children8010048
Pini N, Huo Z, Kym U, Holland-Cunz S, Gros SJ. AQP1-Driven Migration Is Independent of Other Known Adverse Factors but Requires a Hypoxic Undifferentiated Cell Profile in Neuroblastoma. Children. 2021; 8(1):48. https://0-doi-org.brum.beds.ac.uk/10.3390/children8010048
Chicago/Turabian StylePini, Nicola, Zihe Huo, Urs Kym, Stefan Holland-Cunz, and Stephanie J. Gros. 2021. "AQP1-Driven Migration Is Independent of Other Known Adverse Factors but Requires a Hypoxic Undifferentiated Cell Profile in Neuroblastoma" Children 8, no. 1: 48. https://0-doi-org.brum.beds.ac.uk/10.3390/children8010048