The Risk of Nonsteroidal Anti-Inflammatory Drugs in Pediatric Medicine: Listen Carefully to Children with Pain
Abstract
:1. Introduction
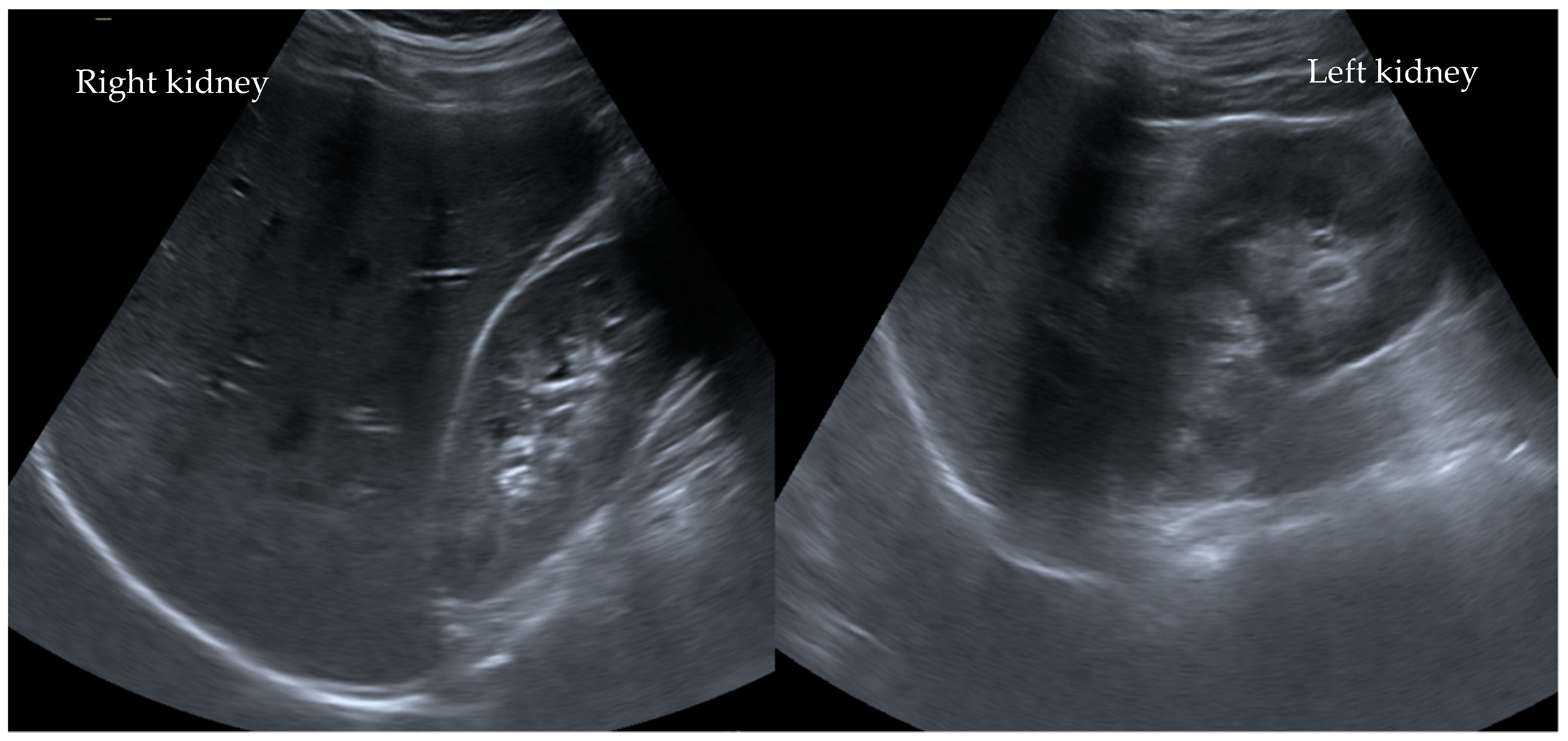
2. Clinical Presentation and Course of Case I°
3. Clinical Presentation and Course of Case II°
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leroy, S.; Mosca, A.; Landre-Peigne, C.; Cosson, M.-A.; Pons, G. Ibuprofen in childhood: Evidence-based review of efficacy and safety. Arch. Pediatr. 2007, 14, 477–484. [Google Scholar] [CrossRef]
- Tan, E.; Braithwaite, I.; McKinlay, C.J.D.; Dalziel, S.R. Comparison of Acetaminophen (Paracetamol) With Ibuprofen for Treatment of Fever or Pain in Children Younger Than 2 Years: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2022398. [Google Scholar] [CrossRef]
- Vernacchio, L.; Kelly, J.P.; Kaufman, D.W.; Mitchell, A.A. Medication use among children <12 years of age in the United States: Results from the Slone Survey. Pediatrics 2009, 124, 446–454. [Google Scholar]
- Yue, Z.; Jiang, P.; Sun, H.; Wu, J. Association between an excess risk of acute kidney injury and concomitant use of ibuprofen and acetaminophen in children, retrospective analysis of a spontaneous reporting system. Eur J. Clin. Pharm. 2014, 70, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Balestracci, A.; Ezquer, M.; Elmo, M.E.; Molini, A.; Thorel, C.; Torrents, M.; Toledo, I. Ibuprofen-associated acute kidney injury in dehydrated children with acute gastroenteritis. Pediatr. Nephrol. 2015, 30, 1873–1878. [Google Scholar] [CrossRef]
- Goyal, A.; Daneshpajouhnejad, P.; Hashmi, M.F.; Bashir, K. Acute Kidney Injury. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Lucas, G.N.C.; Leitão, A.C.C.; Alencar, R.L.; Xavier, R.M.F.; Daher, E.D.F.; da Silva, G.B. Pathophysiological aspects of nephropathy caused by non-steroidal anti-inflammatory drugs. J. Bras. Nefrol. 2019, 41, 124–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hörl, W.H. Nonsteroidal Anti-Inflammatory Drugs and the Kidney. Pharmaceuticals 2010, 3, 2291–2321. [Google Scholar] [CrossRef]
- Praga, M.; González, E. Acute interstitial nephritis. Kidney Int. 2010, 77, 956–961. [Google Scholar] [CrossRef] [Green Version]
- de Martino, M.; Chiarugi, A.; Boner, A.; Montini, G.; de’ Angelis, G.L. Working Towards an Appropriate Use of Ibuprofen in Children: An Evidence-Based Appraisal. Drugs 2017, 77, 1295–1311. [Google Scholar] [CrossRef]
- Ershad, M.; Ameer, M.A.; Vearrier, D. Ibuprofen Toxicity. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Hunter, L.J.; Wood, D.M.; Dargan, P.I. The patterns of toxicity and management of acute nonsteroidal anti-inflammatory drug (NSAID) overdose. Open Access Emerg. Med. 2011, 3, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.H.; Smolinske, S.C.; Conrad, F.L.; Wruk, K.M.; Kulig, K.W.; Dwelle, T.L.; Rumack, B.H. Ibuprofen overdose: 126 cases. Ann. Emerg. Med. 1986, 15, 1308–1313. [Google Scholar] [CrossRef]
- Chung, E.Y.; Tat, S.T. Nonsteroidal Anti-Inflammatory Drug Toxicity in Children: A Clinical Review. Pediatr. Emerg. Care 2016, 32, 250–253. [Google Scholar] [CrossRef]
- Sethi, S.K.; Bunchman, T.; Chakraborty, R.; Raina, R. Pediatric acute kidney injury: New advances in the last decade. Kidney Res. Clin. Pract. 2021, 40, 40–51. [Google Scholar] [CrossRef]
- Dixit, M.; Doan, T.; Kirschner, R.; Dixit, N. Significant Acute Kidney Injury Due to Non-steroidal Anti-inflammatory Drugs: Inpatient Setting. Pharmaceuticals 2010, 3, 1279–1285. [Google Scholar] [CrossRef] [Green Version]
- Whelton, A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: Physiologic foundations and clinical implications. Am. J. Med. 1999, 106, 13S–24S. [Google Scholar] [CrossRef]
- Ravnskov, U. Glomerular, tubular and interstitial nephritis associated with non-steroidal antiinflammatory drugs. Evidence of a common mechanism. Br. J. Clin. Pharm. 1999, 47, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Ulinski, T.; Guigonis, V.; Dunan, O.; Bensman, A. Acute renal failure after treatment with non-steroidal anti-inflammatory drugs. Eur. J. Pediatr. 2004, 163, 148–150. [Google Scholar] [CrossRef]
- Soler, Y.A.; Nieves-Plaza, M.; Prieto, M.; García-De Jesús, R.; Suárez-Rivera, M. pRIFLE (Pediatric Risk, Injury, Failure, Loss, End Stage Renal Disease) score identifies Acute Kidney Injury and predicts mortality in critically ill children: A prospective study. Pediatr. Crit. Care Med. 2013, 14, e189–e195. [Google Scholar] [CrossRef] [Green Version]
- Ciccia, E.; Devarajan, P. Pediatric acute kidney injury: Prevalence, impact and management challenges. Int. J. Nephrol. Renov. Dis. 2017, 10, 77–84. [Google Scholar] [CrossRef] [Green Version]
- Kodner, C.; Kudrimoti, A. Diagnosis and Management of Acute Interstitial Nephritis. AFP 2003, 67, 2527–2534. [Google Scholar]
- Misurac, J.M.; Knoderer, C.A.; Leiser, J.D.; Nailescu, C.; Wilson, A.C.; Andreoli, S.P. Nonsteroidal Anti-Inflammatory Drugs Are an Important Cause of Acute Kidney Injury in Children. J. Pediatr. 2013, 162, 1153–1159.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker-Cohen, R.; Frishberg, Y. Severe reversible renal failure due to naproxen-associated acute interstitial nephritis. Eur J. Pediatr. 2001, 160, 293–295. [Google Scholar] [CrossRef] [PubMed]
- John, C.M.; Shukla, R.; Jones, C.A. Using NSAID in volume depleted children can precipitate acute renal failure. Arch. Dis Child. 2007, 92, 524–526. [Google Scholar] [CrossRef] [PubMed]




| Age- and Sex-Adjusted Reference Range * | Admission | Discharge | |
|---|---|---|---|
| Leukocytes (/nL) | 4.3–10.0 | 13.3 | 7.6 |
| Hemoglobin (g/dL) | 14.0–18.9 | 16.0 | 16.9 |
| Erythrocytes (/pL) | 4.5–5.9 | 4.8 | 5.1 |
| Hematocrit (%) | 41.0–53.0 | 43.8 | 45.6 |
| MCV (fL) | 80–96 | 90.7 | 90.3 |
| MCH (pg) | 28–33 | 33.1 | 33.5 |
| Thrombocytes (/nL) | 150–350 | 217 | 256 |
| GPT (U/I) | 10–50 | 23 | - |
| GGT (U/I) | <66 | 32 | - |
| Lactate (mmol/L) | 1.5 | - | |
| CK-MB (U/I) | <24 | 13 | - |
| Potassium (mmol/L) | 3.5–5.1 | 6.3 | 4.2 |
| Sodium (mmol/L) | 135–145 | 136 | 139 |
| Chlorid (mmol/L) | 96–106 | 104 | 99 |
| Calcium (mmol/L) | - | 2.3 | 2.62 |
| Creatinine (mg/dL) | 0.6–1.3 | 2.4 | 1.2 |
| GFR (mL/min/1.73 m2) | - | 39 | 87 |
| Urea (mg/dL) | <50 | 44 | 43 |
| CRP (mg/dL) | <0.5 | 4.0 | 0.6 |
| Staging | Creatinine Criteria | Urine Output Criteria | |
|---|---|---|---|
| pRIFLE | Risk (R) | eGFR decrease by ≥25% * | <0.5 mL/kg/h for 8 h |
| Injury (I) | eGFR decrease by ≥50% * | <0.5 mL/kg/h for 16 h | |
| Failure (F) | eGFR decrease by ≥75% or <35 mL/min/1.73 m2 * | <0.3 mL/kg/h for 24 h or anuric for 12 h | |
| Loss (L) | Loss of renal function > 4 weeks | ||
| End-Stage (E) | End Stage Renal Disease (persistent failure > 3 months) | ||
| KDIGO | 1 | SCr rise ≥ 0.3 mg/dL within 48 h or an increase in creatinine of ≥50% within 7 day | >0.5 and ≤ 1 mL/kg/h |
| 2 | Increase in creatinine of ≥100% | >0.3 and ≤0.5 mL/kg/h | |
| 3 | Increase in creatinine of ≥200% or SCr ≥ 4 mg/dL or receipt of dialysis or eGFR < 35 mL/min/1.73 m2 | <0.3 mL/kg/h | |
| Age- and Sex-Adjusted Reference Range * | Admission | Discharge | |
|---|---|---|---|
| Leukocytes (/nL) | 4.3–10.0 | 11.6 | 8.4 |
| Hemoglobin (g/dL) | 12.0–16.0 | 12.7 | 13.6 |
| Erythrocytes (/pL) | 4.0–5.2 | 4.3 | 4.7 |
| Hematocrit (%) | 36.0–46.0 | 36.5 | 40.1 |
| MCV (fL) | 80–96 | 84.3 | 86.2 |
| MCH (pg) | 28–33 | 29.3 | 29.2 |
| Thrombocytes (/nL) | 150–350 | 297 | 327 |
| Potassium (mmol/L) | 3.5–5.1 | 7.4 | 4.3 |
| Sodium (mmol/L) | 135–145 | 139 | 140 |
| Chlorid (mmol/L) | 96–106 | 110 | 105 |
| Calcium (mmol/L) | - | 1.7 | 2.62 |
| Creatinine (mg/dL) | 0.5–1.1 | 3.3 | 1.3 |
| GFR (ml/min/1.73 m2) | - | 20 | 57 |
| Urea (mg/dL) | <50 | 31 | 43 |
| CRP (mg/dL) | <0.5 | 4.5 | 0.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mboma, O.; Wirth, S.; Aydin, M. The Risk of Nonsteroidal Anti-Inflammatory Drugs in Pediatric Medicine: Listen Carefully to Children with Pain. Children 2021, 8, 1048. https://0-doi-org.brum.beds.ac.uk/10.3390/children8111048
Mboma O, Wirth S, Aydin M. The Risk of Nonsteroidal Anti-Inflammatory Drugs in Pediatric Medicine: Listen Carefully to Children with Pain. Children. 2021; 8(11):1048. https://0-doi-org.brum.beds.ac.uk/10.3390/children8111048
Chicago/Turabian StyleMboma, Olivier, Stefan Wirth, and Malik Aydin. 2021. "The Risk of Nonsteroidal Anti-Inflammatory Drugs in Pediatric Medicine: Listen Carefully to Children with Pain" Children 8, no. 11: 1048. https://0-doi-org.brum.beds.ac.uk/10.3390/children8111048






