Long-Term Outcomes and the Post-Intensive Care Syndrome in Critically Ill Children: A North American Perspective
Abstract
:1. Introduction
2. Post-Intensive Care Syndrome in Pediatrics
3. Physical Manifestations
4. Cognitive Manifestations
5. Emotional and Psychological Manifestations
6. Social Manifestations and PICs-Family
7. Patient Centered Outcomes in PICs-P
8. Screening and Identification of At-Risk Patients
9. Management and Prevention of PICs-P
10. Research Priorities
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations/Acronyms
| ABCDEF | Bundle of Care to improve ICU outcomes further defined in text |
| COS | Core Outcome Set |
| CPCCRN | Collaborative Pediatric Critical Care Research Network |
| DSM5 | Diagnostics and Statistics Manual 5th Edition |
| ECMO | Extracorporeal Membrane Oxygenation |
| E-CPR | Extracorporeal Cardiopulmonary Resuscitation |
| HRQoL | Health-Related Quality of Life |
| ICU | Intensive Care Unit |
| PALISI | Pediatric Acute Lung Injury and Sepsis Investigators Network |
| PICs | Post-intensive Care Syndrome |
| PICs-P | Post-intensive Care Syndrome in Pediatrics |
| PICU | Pediatric Intensive Care Unit |
| PTSD | Post-traumatic Stress Disorder |
| PN | Parenteral Nutrition |
References
- Epstein, D.; Brill, J.E. A history of pediatric critical care medicine. Pediatr. Res. 2005, 58, 987–996. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, J.D.; Pollack, M.M.; Ruttimann, U.E.; Glass, N.L.; Yeh, T.S. Outcome of pediatric patients with multiple organ system failure. Crit. Care Med. 1986, 14, 271–274. [Google Scholar] [CrossRef]
- Pollack, M.M.; Ruttimann, U.E.; Getson, P.R. Accurate prediction of the outcome of pediatric intensive care. A new quantitative method. N. Engl. J. Med. 1987, 316, 134–139. [Google Scholar] [CrossRef]
- Stoll, B.J.; Holman, R.C.; Schuchat, A. Decline in sepsis-associated neonatal and infant deaths in the United States, 1979 through 1994. Pediatrics 1998, 102, e18. [Google Scholar] [CrossRef] [Green Version]
- Namachivayam, P.; Shann, F.; Shekerdemian, L.; Taylor, A.; van Sloten, I.; Delzoppo, C.; Daffey, C.; Butt, W. Three decades of pediatric intensive care: Who was admitted, what happened in intensive care, and what happened afterward. Pediatr. Crit. Care Med. 2010, 11, 549–555. [Google Scholar] [CrossRef]
- Tan, B.; Wong, J.J.; Sultana, R.; Koh, J.; Jit, M.; Mok, Y.H.; Lee, J.H. Global Case-Fatality Rates in Pediatric Severe Sepsis and Septic Shock: A Systematic Review and Meta-analysis. JAMA Pediatr. 2019, 173, 352–362. [Google Scholar] [CrossRef]
- Watson, R.S.; Crow, S.S.; Hartman, M.E.; Lacroix, J.; Odetola, F.O. Epidemiology and Outcomes of Pediatric Multiple Organ Dysfunction Syndrome. Pediatr. Crit. Care Med. 2017, 18, S4–S16. [Google Scholar] [CrossRef] [Green Version]
- Merritt, C.; Menon, K.; Agus, M.S.D.; Choong, K.; McNally, D.; O’Hearn, K.; Watson, R.S.; Wong, H.R.; Duffett, M.; Wypij, D.; et al. Beyond Survival: Pediatric Critical Care Interventional Trial Outcome Measure Preferences of Families and Healthcare Professionals. Pediatr. Crit. Care Med. 2018, 19, e105–e111. [Google Scholar] [CrossRef]
- Pollack, M.M.; Holubkov, R.; Funai, T.; Dean, J.M.; Berger, J.T.; Wessel, D.L.; Meert, K.; Berg, R.A.; Newth, C.J.; Harrison, R.E.; et al. The Pediatric Risk of Mortality Score: Update 2015. Pediatr. Crit. Care Med. 2016, 17, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Wolfler, A.; Osello, R.; Gualino, J.; Calderini, E.; Vigna, G.; Santuz, P.; Amigoni, A.; Savron, F.; Caramelli, F.; Rossetti, E.; et al. The Importance of Mortality Risk Assessment: Validation of the Pediatric Index of Mortality 3 Score. Pediatr. Crit. Care Med. 2016, 17, 251–256. [Google Scholar] [CrossRef]
- Burns, J.P.; Sellers, D.E.; Meyer, E.C.; Lewis-Newby, M.; Truog, R.D. Epidemiology of death in the PICU at five U.S. teaching hospitals*. Crit. Care Med. 2014, 42, 2101–2108. [Google Scholar] [CrossRef] [Green Version]
- Pollack, M.M.; Holubkov, R.; Funai, T.; Clark, A.; Berger, J.T.; Meert, K.; Newth, C.J.; Shanley, T.; Moler, F.; Carcillo, J.; et al. Pediatric intensive care outcomes: Development of new morbidities during pediatric critical care. Pediatr. Crit. Care Med. 2014, 15, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Traube, C.; Silver, G.; Gerber, L.M.; Kaur, S.; Mauer, E.A.; Kerson, A.; Joyce, C.; Greenwald, B.M. Delirium and Mortality in Critically Ill Children: Epidemiology and Outcomes of Pediatric Delirium. Crit. Care Med. 2017, 45, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Creten, C.; Van Der Zwaan, S.; Blankespoor, R.J.; Leroy, P.L.; Schieveld, J.N. Pediatric delirium in the pediatric intensive care unit: A systematic review and an update on key issues and research questions. Minerva Anestesiol. 2011, 77, 1099–1107. [Google Scholar]
- Aspesberro, F.; Mangione-Smith, R.; Zimmerman, J.J. Health-related quality of life following pediatric critical illness. Intensive Care Med. 2015, 41, 1235–1246. [Google Scholar] [CrossRef]
- Rennick, J.E.; Childerhose, J.E. Redefining success in the PICU: New patient populations shift targets of care. Pediatrics 2015, 135, e289–e291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanthimathinathan, H.K.; Plunkett, A.; Scholefield, B.R.; Pearson, G.A.; Morris, K.P. Trends in long-stay admissions to a UK paediatric intensive care unit. Arch. Dis. Child. 2020, 105, 558–562. [Google Scholar] [CrossRef]
- Odetola, F.O.; Gebremariam, A.; Davis, M.M. Comorbid illnesses among critically ill hospitalized children: Impact on hospital resource use and mortality, 1997–2006. Pediatr. Crit. Care Med. 2010, 11, 457–463. [Google Scholar] [CrossRef]
- Edwards, J.D.; Houtrow, A.J.; Vasilevskis, E.E.; Rehm, R.S.; Markovitz, B.P.; Graham, R.J.; Dudley, R.A. Chronic conditions among children admitted to U.S. pediatric intensive care units: Their prevalence and impact on risk for mortality and prolonged length of stay*. Crit. Care Med. 2012, 40, 2196–2203. [Google Scholar] [CrossRef] [Green Version]
- Maddux, A.B.; Pinto, N.; Fink, E.L.; Hartman, M.E.; Nett, S.; Biagas, K.; Killien, E.Y.; Dervan, L.A.; Christie, L.M.; Luckett, P.M.; et al. Postdischarge Outcome Domains in Pediatric Critical Care and the Instruments Used to Evaluate Them: A Scoping Review. Crit. Care Med. 2020, 48, e1313–e1321. [Google Scholar] [CrossRef]
- Needham, D.M.; Davidson, J.; Cohen, H.; Hopkins, R.O.; Weinert, C.; Wunsch, H.; Zawistowski, C.; Bemis-Dougherty, A.; Berney, S.C.; Bienvenu, O.J.; et al. Improving long-term outcomes after discharge from intensive care unit: Report from a stakeholders’ conference. Crit. Care Med. 2012, 40, 502–509. [Google Scholar] [CrossRef]
- Davidson, J.E.; Jones, C.; Bienvenu, O.J. Family response to critical illness: Postintensive care syndrome-family. Crit. Care Med. 2012, 40, 618–624. [Google Scholar] [CrossRef]
- Manning, J.C.; Pinto, N.P.; Rennick, J.E.; Colville, G.; Curley, M.A.Q. Conceptualizing Post Intensive Care Syndrome in Children-The PICS-p Framework. Pediatr. Crit. Care Med. 2018, 19, 298–300. [Google Scholar] [CrossRef]
- Rennick, J.E.; Dougherty, G.; Chambers, C.; Stremler, R.; Childerhose, J.E.; Stack, D.M.; Harrison, D.; Campbell-Yeo, M.; Dryden-Palmer, K.; Zhang, X.; et al. Children’s psychological and behavioral responses following pediatric intensive care unit hospitalization: The caring intensively study. BMC Pediatr. 2014, 14, 276. [Google Scholar] [CrossRef] [Green Version]
- Colville, G.A.; Pierce, C.M. Children’s self-reported quality of life after intensive care treatment. Pediatr. Crit. Care Med. 2013, 14, e85–e92. [Google Scholar] [CrossRef]
- Manning, J.C.; Hemingway, P.; Redsell, S.A. Stories of survival: Children’s narratives of psychosocial well-being following paediatric critical illness or injury. J. Child Health Care 2017, 21, 236–252. [Google Scholar] [CrossRef]
- Colville, G.; Pierce, C. Patterns of post-traumatic stress symptoms in families after paediatric intensive care. Intensive Care Med. 2012, 38, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.; Lee, J.H.; Leow, M.K.; Puthucheary, Z.A. Functional Outcomes and Physical Impairments in Pediatric Critical Care Survivors: A Scoping Review. Pediatr. Crit. Care Med. 2016, 17, e247–e259. [Google Scholar] [CrossRef] [PubMed]
- Osler, W.; McCrae, T. The Principles and Practice of Medicine: Designed for the Use of Practitioners and Students of Medicine, 8th ed.; D. Appleton: New York, NY, USA, 1915; p. 1225. [Google Scholar]
- Olsen, C.W. Lesions of peripheral nerves developing during coma. J. Am. Med. Assoc. 1956, 160, 39–41. [Google Scholar] [CrossRef]
- MacFarlane, I.A.; Rosenthal, F.D. Severe myopathy after status asthmaticus. Lancet 1977, 2, 615. [Google Scholar] [CrossRef]
- Bolton, C.F.; Gilbert, J.J.; Hahn, A.F.; Sibbald, W.J. Polyneuropathy in critically ill patients. J. Neurol. Neurosurg. Psychiatry 1984, 47, 1223–1231. [Google Scholar] [CrossRef]
- Siu, K.; Al-Harbi, S.; Clark, H.; Thabane, L.; Cheng, J.; Tarnopolsky, M.; Meaney, B.; Choong, K. Feasibility and Reliability of Muscle Strength Testing in Critically Ill Children. J. Pediatr. Intensive Care 2015, 4, 218–224. [Google Scholar] [CrossRef]
- Pollack, M.M.; Holubkov, R.; Funai, T.; Clark, A.; Moler, F.; Shanley, T.; Meert, K.; Newth, C.J.; Carcillo, J.; Berger, J.T.; et al. Relationship between the functional status scale and the pediatric overall performance category and pediatric cerebral performance category scales. JAMA Pediatr. 2014, 168, 671–676. [Google Scholar] [CrossRef] [Green Version]
- Pinto, N.P.; Rhinesmith, E.W.; Kim, T.Y.; Ladner, P.H.; Pollack, M.M. Long-Term Function After Pediatric Critical Illness: Results From the Survivor Outcomes Study. Pediatr. Crit. Care Med. 2017, 18, e122–e130. [Google Scholar] [CrossRef]
- Pollack, M.M.; Banks, R.; Holubkov, R.; Meert, K.L.; the Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network. Long-Term Outcome of PICU Patients Discharged with New, Functional Status Morbidity. Pediatr. Crit. Care Med. 2021, 22, 27–39. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Functioning, Disability, and Health: Children & Youth Version: ICF-CY; World Health Organization: Geneva, Switzerland, 2007; p. 322. [Google Scholar]
- Ward, S.L.; Turpin, A.; Spicer, A.C.; Treadwell, M.J.; Church, G.D.; Flori, H.R. Long-Term Pulmonary Function and Quality of Life in Children After Acute Respiratory Distress Syndrome: A Feasibility Investigation. Pediatr. Crit. Care Med. 2017, 18, e48–e55. [Google Scholar] [CrossRef] [Green Version]
- Field-Ridley, A.; Dharmar, M.; Steinhorn, D.; McDonald, C.; Marcin, J.P. ICU-Acquired Weakness Is Associated With Differences in Clinical Outcomes in Critically Ill Children. Pediatr. Crit. Care Med. 2016, 17, 53–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choong, K.; Fraser, D.; Al-Harbi, S.; Borham, A.; Cameron, J.; Cameron, S.; Cheng, J.; Clark, H.; Doherty, T.; Fayed, N.; et al. Functional Recovery in Critically Ill Children, the "WeeCover" Multicenter Study. Pediatr. Crit. Care Med. 2018, 19, 145–154. [Google Scholar] [CrossRef]
- Edwards, J.D.; Lucas, A.R.; Stone, P.W.; Boscardin, W.J.; Dudley, R.A. Frequency, risk factors, and outcomes of early unplanned readmissions to PICUs. Crit. Care Med. 2013, 41, 2773–2783. [Google Scholar] [CrossRef] [Green Version]
- Yagiela, L.M.; Barbaro, R.P.; Quasney, M.W.; Pfarr, M.A.; Ursu, D.C.; Prosser, L.A.; Odetola, F.O. Outcomes and Patterns of Healthcare Utilization After Hospitalization for Pediatric Critical Illness Due to Respiratory Failure. Pediatr. Crit. Care Med. 2019, 20, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.D.; Lucas, A.R.; Boscardin, W.J.; Dudley, R.A. Repeated Critical Illness and Unplanned Readmissions Within 1 Year to PICUs. Crit. Care Med. 2017, 45, 1276–1284. [Google Scholar] [CrossRef]
- Pollack, M.M.; Holubkov, R.; Glass, P.; Dean, J.M.; Meert, K.L.; Zimmerman, J.; Anand, K.J.; Carcillo, J.; Newth, C.J.; Harrison, R.; et al. Functional Status Scale: New pediatric outcome measure. Pediatrics 2009, 124, e18–e28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pollack, M.M.; Holubkov, R.; Funai, T.; Berger, J.T.; Clark, A.E.; Meert, K.; Berg, R.A.; Carcillo, J.; Wessel, D.L.; Moler, F.; et al. Simultaneous Prediction of New Morbidity, Mortality, and Survival Without New Morbidity From Pediatric Intensive Care: A New Paradigm for Outcomes Assessment. Crit. Care Med. 2015, 43, 1699–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bone, M.F.; Feinglass, J.M.; Goodman, D.M. Risk factors for acquiring functional and cognitive disabilities during admission to a PICU*. Pediatr. Crit. Care Med. 2014, 15, 640–648. [Google Scholar] [CrossRef]
- Watson, R.S.; Asaro, L.A.; Hutchins, L.; Bysani, G.K.; Killien, E.Y.; Angus, D.C.; Wypij, D.; Curley, M.A.Q. Risk Factors for Functional Decline and Impaired Quality of Life after Pediatric Respiratory Failure. Am. J. Respir. Crit. Care Med. 2019, 200, 900–909. [Google Scholar] [CrossRef]
- Watson, R.S.; Asaro, L.A.; Hertzog, J.H.; Sorce, L.R.; Kachmar, A.G.; Dervan, L.A.; Angus, D.C.; Wypij, D.; Curley, M.A.Q.; Investigators, R.S.; et al. Long-Term Outcomes after Protocolized Sedation versus Usual Care in Ventilated Pediatric Patients. Am. J. Respir. Crit. Care Med. 2018, 197, 1457–1467. [Google Scholar] [CrossRef]
- Farris, R.W.; Weiss, N.S.; Zimmerman, J.J. Functional outcomes in pediatric severe sepsis: Further analysis of the researching severe sepsis and organ dysfunction in children: A global perspective trial. Pediatr. Crit. Care Med. 2013, 14, 835–842. [Google Scholar] [CrossRef] [Green Version]
- Bennett, T.D.; Dixon, R.R.; Kartchner, C.; DeWitt, P.E.; Sierra, Y.; Ladell, D.; Kempe, A.; Runyan, D.K.; Dean, J.M.; Keenan, H.T. Functional Status Scale in Children With Traumatic Brain Injury: A Prospective Cohort Study. Pediatr. Crit. Care Med. 2016, 17, 1147–1156. [Google Scholar] [CrossRef] [Green Version]
- Sankar, J.; Moodu, S.; Kumar, K.; Sankar, M.J.; Kabra, S.K.; Lodha, R. Functional Outcomes at 1 Year After PICU Discharge in Critically Ill Children With Severe Sepsis. Pediatr. Crit. Care Med. 2021, 22, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Slovis, J.C.; Gupta, N.; Li, N.Y.; Kernie, S.G.; Miles, D.K. Assessment of Recovery Following Pediatric Traumatic Brain Injury. Pediatr. Crit. Care Med. 2018, 19, 353–360. [Google Scholar] [CrossRef]
- Heneghan, J.A.; Reeder, R.W.; Dean, J.M.; Meert, K.L.; Berg, R.A.; Carcillo, J.; Newth, C.J.L.; Dalton, H.; Tamburro, R.; Pollack, M.M. Characteristics and Outcomes of Critical Illness in Children With Feeding and Respiratory Technology Dependence. Pediatr. Crit. Care Med. 2019, 20, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Als, L.C.; Nadel, S.; Cooper, M.; Pierce, C.M.; Sahakian, B.J.; Garralda, M.E. Neuropsychologic function three to six months following admission to the PICU with meningoencephalitis, sepsis, and other disorders: A prospective study of school-aged children. Crit. Care Med. 2013, 41, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Elias, M.D.; Achuff, B.J.; Ittenbach, R.F.; Ravishankar, C.; Spray, T.L.; Fuller, S.; Montenegro, L.M.; Gaynor, J.W.; O’Connor, M.J. Long-Term Outcomes of Pediatric Cardiac Patients Supported by Extracorporeal Membrane Oxygenation. Pediatr. Crit. Care Med. 2017, 18, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.S.; Choong, K.; Colville, G.; Crow, S.; Dervan, L.A.; Hopkins, R.O.; Knoester, H.; Pollack, M.M.; Rennick, J.; Curley, M.A.Q. Life after Critical Illness in Children-Toward an Understanding of Pediatric Post-intensive Care Syndrome. J. Pediatr. 2018, 198, 16–24. [Google Scholar] [CrossRef]
- Vermunt, L.C.; Buysse, C.M.; Joosten, K.F.; Duivenvoorden, H.J.; Hazelzet, J.A.; Verhulst, F.C.; Utens, E.M. Survivors of septic shock caused by Neisseria meningitidis in childhood: Psychosocial outcomes in young adulthood. Pediatr. Crit. Care Med. 2011, 12, e302–e309. [Google Scholar] [CrossRef]
- Elison, S.; Shears, D.; Nadel, S.; Sahakian, B.; Garralda, M.E. Neuropsychological function in children following admission to paediatric intensive care: A pilot investigation. Intensive Care Med. 2008, 34, 1289–1293. [Google Scholar] [CrossRef]
- Kachmar, A.G.; Irving, S.Y.; Connolly, C.A.; Curley, M.A.Q. A Systematic Review of Risk Factors Associated With Cognitive Impairment After Pediatric Critical Illness. Pediatr. Crit. Care Med. 2018, 19, e164–e171. [Google Scholar] [CrossRef]
- Garcia Guerra, G.; Zorzela, L.; Robertson, C.M.; Alton, G.Y.; Joffe, A.R.; Moez, E.K.; Dinu, I.A.; Ross, D.B.; Rebeyka, I.M.; Lequier, L.; et al. Survival and neurocognitive outcomes in pediatric extracorporeal-cardiopulmonary resuscitation. Resuscitation 2015, 96, 208–213. [Google Scholar] [CrossRef]
- Als, L.C.; Picouto, M.D.; Hau, S.M.; Nadel, S.; Cooper, M.; Pierce, C.M.; Kramer, T.; Garralda, M.E. Mental and physical well-being following admission to pediatric intensive care. Pediatr. Crit. Care Med. 2015, 16, e141–e149. [Google Scholar] [CrossRef] [PubMed]
- Mestrovic, J.; Kardum, G.; Sustic, A.; Polic, B.; Mestrovic, M.; Markic, J.; Zanchi, J. Neurodevelopmental disabilities and quality of life after intensive care treatment. J. Paediatr. Child Health 2007, 43, 673–676. [Google Scholar] [CrossRef]
- Diagnostic and Statistics Manual of Mental Disorders, 5th ed.; American Psychatic Association: Arlington, VA, USA, 2013.
- Davydow, D.S.; Richardson, L.P.; Zatzick, D.F.; Katon, W.J. Psychiatric morbidity in pediatric critical illness survivors: A comprehensive review of the literature. Arch. Pediatr. Adolesc. Med. 2010, 164, 377–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rennick, J.E.; Rashotte, J. Psychological outcomes in children following pediatric intensive care unit hospitalization: A systematic review of the research. J. Child Health Care 2009, 13, 128–149. [Google Scholar] [CrossRef]
- Carnevale, F.A. The experience of critically ill children: Narratives of unmaking. Intensive Crit. Care Nurs. 1997, 13, 49–52. [Google Scholar] [CrossRef]
- Colville, G.; Kerry, S.; Pierce, C. Children’s factual and delusional memories of intensive care. Am. J. Respir. Crit. Care Med. 2008, 177, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Rennick, J.E.; McHarg, L.F.; Dell’Api, M.; Johnston, C.C.; Stevens, B. Developing the Children’s Critical Illness Impact Scale: Capturing stories from children, parents, and staff. Pediatr. Crit. Care Med. 2008, 9, 252–260. [Google Scholar] [CrossRef]
- Lopes-Junior, L.C.; Rosa, M.; Lima, R.A.G. Psychological and Psychiatric Outcomes Following PICU Admission: A Systematic Review of Cohort Studies. Pediatr. Crit. Care Med. 2018, 19, e58–e67. [Google Scholar] [CrossRef]
- Rees, G.; Gledhill, J.; Garralda, M.E.; Nadel, S. Psychiatric outcome following paediatric intensive care unit (PICU) admission: A cohort study. Intensive Care Med. 2004, 30, 1607–1614. [Google Scholar] [CrossRef]
- Rady, H.I.; Ismail, O.R.; Abdelkader, M.S.; Abdelgalil, A.A. Increased Psychiatric Risk in Children After Pediatric Intensive Care Unit Admission. J. Nerv. Ment. Dis. 2020, 208, 147–151. [Google Scholar] [CrossRef]
- Nelson, L.P.; Lachman, S.E.; Li, S.W.; Gold, J.I. The Effects of Family Functioning on the Development of Posttraumatic Stress in Children and Their Parents Following Admission to the PICU. Pediatr. Crit. Care Med. 2019, 20, e208–e215. [Google Scholar] [CrossRef]
- Colville, G.A.; Pierce, C.M.; Peters, M.J. Self-Reported Fatigue in Children Following Intensive Care Treatment. Pediatr. Crit. Care Med. 2019, 20, e98–e101. [Google Scholar] [CrossRef]
- Hordijk, J.; Verbruggen, S.; Vanhorebeek, I.; Guiza, F.; Wouters, P.; Van den Berghe, G.; Joosten, K.; Dulfer, K. Health-related quality of life of children and their parents 2 years after critical illness: Pre-planned follow-up of the PEPaNIC international, randomized, controlled trial. Crit. Care 2020, 24, 347. [Google Scholar] [CrossRef] [PubMed]
- Hordijk, J.; Verbruggen, S.; Vanhorebeek, I.; Van den Berghe, G.; Utens, E.; Joosten, K.; Dulfer, K. Health-related quality of life of children and their parents 6 months after children’s critical illness. Qual. Life Res. 2020, 29, 179–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, S.C.; Gledhill, J.A. Systematic Review of Interventions to Reduce Psychiatric Morbidity in Parents and Children After PICU Admissions. Pediatr. Crit. Care Med. 2017, 18, 343–348. [Google Scholar] [CrossRef]
- Atkins, E.K.; John, M.; Colville, G. Families’ Experiences of Life in the Year after a Child’s Critical Illness: Navigating the Road to a “New Normal”. J. Pediatr. Intensive Care 2020, 9, 188–195. [Google Scholar] [CrossRef]
- Foster, K.; Mitchell, R.; Van, C.; Young, A.; McCloughen, A.; Curtis, K. Resilient, recovering, distressed: A longitudinal qualitative study of parent psychosocial trajectories following child critical injury. Injury 2019, 50, 1605–1611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, K.; Mitchell, R.; Young, A.; Van, C.; Curtis, K. Parent experiences and psychosocial support needs 6 months following paediatric critical injury: A qualitative study. Injury 2019, 50, 1082–1088. [Google Scholar] [CrossRef]
- Allison, M.A.; Attisha, E.; Council On School, H. The Link Between School Attendance and Good Health. Pediatrics 2019, 143. [Google Scholar] [CrossRef] [Green Version]
- Kastner, K.; Pinto, N.; Msall, M.E.; Sobotka, S. PICU Follow-Up: The Impact of Missed School in a Cohort of Children Following PICU Admission. Crit. Care Explor. 2019, 1, e0033. [Google Scholar] [CrossRef]
- Emerson, N.D.; Distelberg, B.; Morrell, H.E.; Williams-Reade, J.; Tapanes, D.; Montgomery, S. Quality of Life and School Absenteeism in Children With Chronic Illness. J. Sch. Nurs. 2016, 32, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, M.C.; Henderson, C.M.; Hutton, N.; Boss, R.D. Defining Pediatric Chronic Critical Illness for Clinical Care, Research, and Policy. Hosp. Pediatr. 2017, 7, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Rey, R.; Alonso-Tapia, J.; Colville, G. Prediction of parental posttraumatic stress, anxiety and depression after a child’s critical hospitalization. J. Crit. Care 2018, 45, 149–155. [Google Scholar] [CrossRef]
- Nelson, L.P.; Gold, J.I. Posttraumatic stress disorder in children and their parents following admission to the pediatric intensive care unit: A review. Pediatr. Crit. Care Med. 2012, 13, 338–347. [Google Scholar] [CrossRef]
- Logan, G.E.; Sahrmann, J.M.; Gu, H.; Harman, M. Parental Mental Health Care After Their Child’s Pediatric Intesnive Care Hospitaliation. Pediatr. Crit. Care Med. 2020, 21, 941–948. [Google Scholar] [CrossRef] [PubMed]
- Bronner, M.B.; Peek, N.; Knoester, H.; Bos, A.P.; Last, B.F.; Grootenhuis, M.A. Course and predictors of posttraumatic stress disorder in parents after pediatric intensive care treatment of their child. J. Pediatr. Psychol. 2010, 35, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.S.P.; Grossoehme, D.H.; Forbes, M.L.; Friebert, S. Provider Consensus on Candidate Protective and Risk Factors for Adverse Psychosocial Outcomes Following Discharge From a PICU: A Modified Delphi Study. Pediatr. Crit. Care Med. 2020, 21, e1–e7. [Google Scholar] [CrossRef]
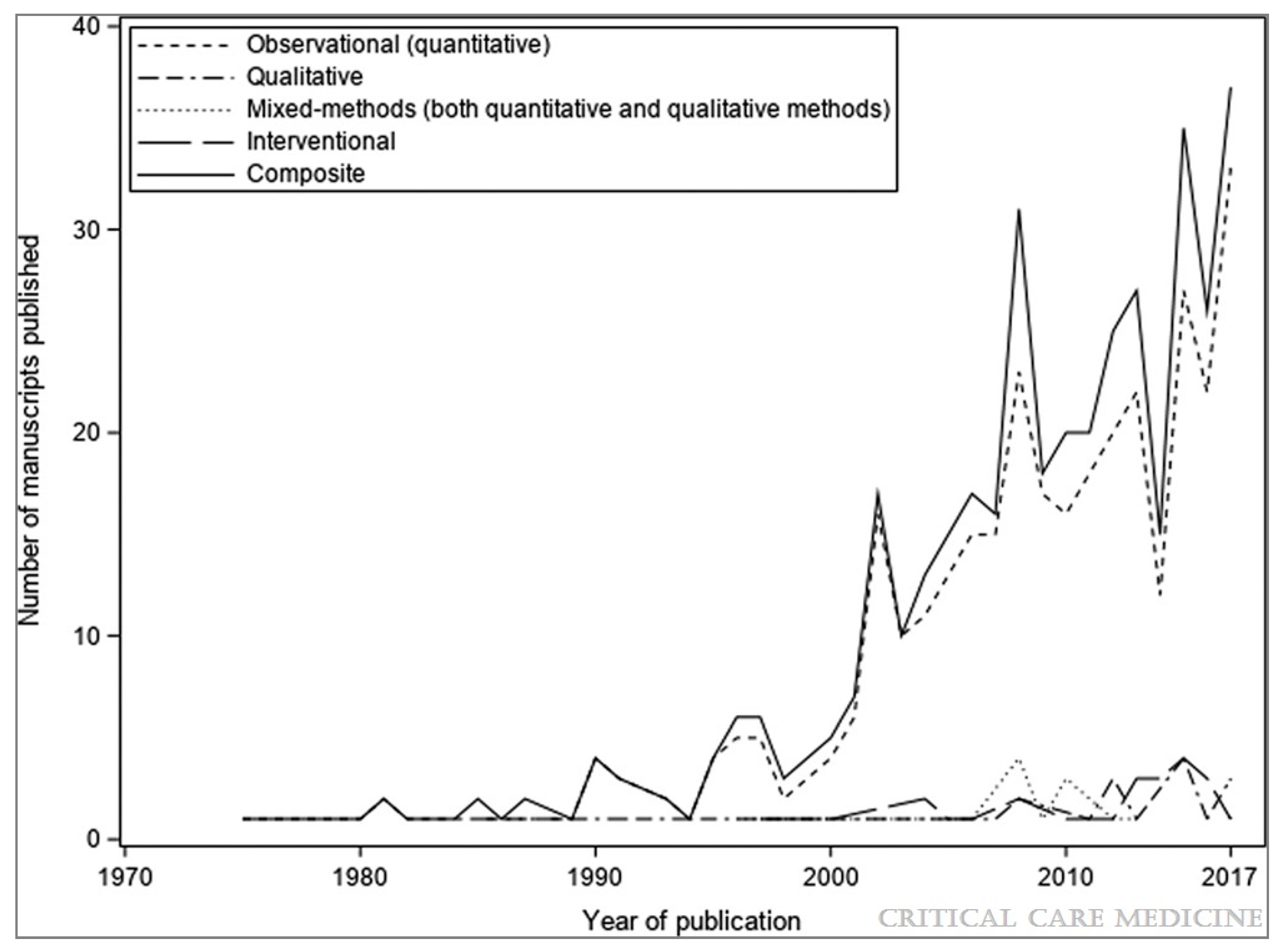
- Duffett, M.; Choong, K.; Hartling, L.; Menon, K.; Thabane, L.; Cook, D.J. Randomized controlled trials in pediatric critical care: A scoping review. Crit. Care 2013, 17, R256. [Google Scholar] [CrossRef] [Green Version]
- Aspesberro, F.; Fesinmeyer, M.D.; Zhou, C.; Zimmerman, J.J.; Mangione-Smith, R. Construct Validity and Responsiveness of the Pediatric Quality of Life Inventory 4.0 Generic Core Scales and Infant Scales in the PICU. Pediatr. Crit. Care Med. 2016, 17, e272–e279. [Google Scholar] [CrossRef]
- Fayed, N.; Cameron, S.; Fraser, D.; Cameron, J.I.; Al-Harbi, S.; Simpson, R.; Wakim, M.; Chiu, L.; Choong, K. Priority Outcomes in Critically Ill Children: A Patient and Parent Perspective. Am. J. Crit. Care 2020, 29, e94–e103. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, S.; Singh, S.; Hutchison, J.S.; Kulkarni, A.V.; Sananes, R.; Bowman, K.W.; Parshuram, C.S. Adaptive behavior, functional outcomes, and quality of life outcomes of children requiring urgent ICU admission. Pediatr. Crit. Care Med. 2013, 14, 10–18. [Google Scholar] [CrossRef]
- Buysse, C.M.; Vermunt, L.C.; Raat, H.; Hazelzet, J.A.; Hop, W.C.; Utens, E.M.; Joosten, K.F. Surviving meningococcal septic shock in childhood: Long-term overall outcome and the effect on health-related quality of life. Crit. Care 2010, 14, R124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edmond, K.; Dieye, Y.; Griffiths, U.K.; Fleming, J.; Ba, O.; Diallo, N.; Mulholland, K. Prospective cohort study of disabling sequelae and quality of life in children with bacterial meningitis in urban Senegal. Pediatr. Infect. Dis. J. 2010, 29, 1023–1029. [Google Scholar] [CrossRef]
- Martin-Herz, S.P.; Zatzick, D.F.; McMahon, R.J. Health-related quality of life in children and adolescents following traumatic injury: A review. Clin. Child Fam. Psychol. Rev. 2012, 15, 192–214. [Google Scholar] [CrossRef] [Green Version]
- Polic, B.; Mestrovic, J.; Markic, J.; Mestrovic, M.; Capkun, V.; Utrobicic, I.; Jukica, M.; Radonic, M. Long-term quality of life of patients treated in paediatric intensive care unit. Eur. J. Pediatr. 2013, 172, 85–90. [Google Scholar] [CrossRef]
- Namachivayam, P.; Taylor, A.; Montague, T.; Moran, K.; Barrie, J.; Delzoppo, C.; Butt, W. Long-stay children in intensive care: Long-term functional outcome and quality of life from a 20-yr institutional study. Pediatr. Crit. Care Med. 2012, 13, 520–528. [Google Scholar] [CrossRef] [Green Version]
- Fink, E.L.; Jarvis, J.M.; Maddux, A.B.; Pinto, N.; Galyean, P.; Olson, L.M.; Zickmund, S.; Ringwood, M.; Sorenson, S.; Dean, J.M.; et al. Development of a core outcome set for pediatric critical care outcomes research. Contemp. Clin. Trials 2020, 91, 105968. [Google Scholar] [CrossRef] [PubMed]
- Fink, E.L.; Maddux, A.B.; Pinto, N.; Sorenson, S.; Notterman, D.; Dean, J.M.; Carcillo, J.A.; Berg, R.A.; Zuppa, A.; Pollack, M.M.; et al. A Core Outcome Set for Pediatric Critical Care. Crit. Care Med. 2020, 48, 1819–1828. [Google Scholar] [CrossRef]
- Matics, T.J.; Pinto, N.P.; Sanchez-Pinto, L.N. Association of Organ Dysfunction Scores and Functional Outcomes Following Pediatric Critical Illness. Pediatr. Crit. Care Med. 2019, 20, 722–727. [Google Scholar] [CrossRef]
- Senna, S.; Ong, C.; Wong, J.J.; Allen, J.C., Jr.; Sultana, R.; Lee, J.H. Prediction of Acquired Morbidity Using Illness Severity Indices in Pediatric Intensive Care Patients. Pediatr. Crit. Care Med. 2020, 21, e972–e980. [Google Scholar] [CrossRef] [PubMed]
- Meert, K.L.; Reeder, R.; Maddux, A.B.; Banks, R.; Berg, R.A.; Zuppa, A.; Newth, C.J.; Wessel, D.; Pollack, M.M.; Hall, M.W.; et al. Trajectories and Risk Factors for Altered Physical and Psychosocial Health-Related Quality of Life After Pediatric Community-Acquired Septic Shock. Pediatr. Crit. Care Med. 2020, 21, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Silver, G.; Doyle, H.; Hegel, E.; Kaur, S.; Mauer, E.A.; Gerber, L.M.; Traube, C. Association Between Pediatric Delirium and Quality of Life After Discharge. Crit. Care Med. 2020, 48, 1829–1834. [Google Scholar] [CrossRef]
- Devlin, J.W.; Skrobik, Y.; Gelinas, C.; Needham, D.M.; Slooter, A.J.C.; Pandharipande, P.P.; Watson, P.L.; Weinhouse, G.L.; Nunnally, M.E.; Rochwerg, B.; et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit. Care Med. 2018, 46, e825–e873. [Google Scholar] [CrossRef] [Green Version]
- Choong, K.; Tran, N.; Clark, H.; Cupido, C.; Corsi, D.J. Acute rehabilitation in critically ill children. J. Pediatr. Intensive Care 2012, 1, 183–192. [Google Scholar] [CrossRef]
- van Zellem, L.; Utens, E.M.; de Wildt, S.N.; Vet, N.J.; Tibboel, D.; Buysse, C. Analgesia-sedation in PICU and neurological outcome: A secondary analysis of long-term neuropsychological follow-up in meningococcal septic shock survivors*. Pediatr. Crit. Care Med. 2014, 15, 189–196. [Google Scholar] [CrossRef]
- Dervan, L.A.; Di Gennaro, J.L.; Farris, R.W.D.; Watson, R.S. Delirium in a Tertiary PICU: Risk Factors and Outcomes. Pediatr. Crit. Care Med. 2020, 21, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Meyburg, J.; Ries, M.; Zielonka, M.; Koch, K.; Sander, A.; von Haken, R.; Reuner, G. Cognitive and Behavioral Consequences of Pediatric Delirium: A Pilot Study. Pediatr. Crit. Care Med. 2018, 19, e531–e537. [Google Scholar] [CrossRef] [PubMed]
- Verstraete, S.; Verbruggen, S.C.; Hordijk, J.A.; Vanhorebeek, I.; Dulfer, K.; Guiza, F.; van Puffelen, E.; Jacobs, A.; Leys, S.; Durt, A.; et al. Long-term developmental effects of withholding parenteral nutrition for 1 week in the paediatric intensive care unit: A 2-year follow-up of the PEPaNIC international, randomised, controlled trial. Lancet Respir. Med. 2019, 7, 141–153. [Google Scholar] [CrossRef]
- Jacobs, A.; Dulfer, K.; Eveleens, R.D.; Hordijk, J.; Van Cleemput, H.; Verlinden, I.; Wouters, P.J.; Mebis, L.; Guerra, G.G.; Joosten, K.; et al. Long-term developmental effect of withholding parenteral nutrition in paediatric intensive care units: A 4-year follow-up of the PEPaNIC randomised controlled trial. Lancet Child Adolesc. Health 2020, 4, 503–514. [Google Scholar] [CrossRef]
- Biagas, K.V.; Hinton, V.J.; Hasbani, N.R.; Luckett, P.M.; Wypij, D.; Nadkarni, V.M.; Agus, M.S.D.; HALF-PINT Trial Study Investigators; Network, P. Long-Term Neurobehavioral and Quality of Life Outcomes of Critically Ill Children after Glycemic Control. J. Pediatr. 2020, 218, 57–63.e55. [Google Scholar] [CrossRef] [Green Version]
- Meert, K.; Slomine, B.S.; Christensen, J.R.; Telford, R.; Holubkov, R.; Dean, J.M.; Moler, F.W. Burden of caregiving after a child’s in-hospital cardiac arrest. Resuscitation 2018, 127, 44–50. [Google Scholar] [CrossRef]
- Treble-Barna, A.; Beers, S.R.; Houtrow, A.J.; Ortiz-Aguayo, R.; Valenta, C.; Stanger, M.; Chrisman, M.; Orringer, M.; Smith, C.M.; Pollon, D.; et al. PICU-Based Rehabilitation and Outcomes Assessment: A Survey of Pediatric Critical Care Physicians. Pediatr. Crit. Care Med. 2019, 20, e274–e282. [Google Scholar] [CrossRef]
- Jackson, J.C.; Santoro, M.J.; Ely, T.M.; Boehm, L.; Kiehl, A.L.; Anderson, L.S.; Ely, E.W. Improving patient care through the prism of psychology: Application of Maslow’s hierarchy to sedation, delirium, and early mobility in the intensive care unit. J. Crit. Care 2014, 29, 438–444. [Google Scholar] [CrossRef] [Green Version]
- Morandi, A.; Piva, S.; Ely, E.W.; Myatra, S.N.; Salluh, J.I.F.; Amare, D.; Azoulay, E.; Bellelli, G.; Csomos, A.; Fan, E.; et al. Worldwide Survey of the “Assessing Pain, Both Spontaneous Awakening and Breathing Trials, Choice of Drugs, Delirium Monitoring/Management, Early Exercise/Mobility, and Family Empowerment” (ABCDEF) Bundle. Crit. Care Med. 2017, 45, e1111–e1122. [Google Scholar] [CrossRef] [PubMed]
- Pun, B.T.; Balas, M.C.; Barnes-Daly, M.A.; Thompson, J.L.; Aldrich, J.M.; Barr, J.; Byrum, D.; Carson, S.S.; Devlin, J.W.; Engel, H.J.; et al. Caring for Critically Ill Patients with the ABCDEF Bundle: Results of the ICU Liberation Collaborative in Over 15,000 Adults. Crit. Care Med. 2019, 47, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Cuello-Garcia, C.A.; Mai, S.H.C.; Simpson, R.; Al-Harbi, S.; Choong, K. Early Mobilization in Critically Ill Children: A Systematic Review. J. Pediatr. 2018, 203, 25–33.e26. [Google Scholar] [CrossRef] [Green Version]
- Curley, M.A.; Wypij, D.; Watson, R.S.; Grant, M.J.; Asaro, L.A.; Cheifetz, I.M.; Dodson, B.L.; Franck, L.S.; Gedeit, R.G.; Angus, D.C.; et al. Protocolized sedation vs usual care in pediatric patients mechanically ventilated for acute respiratory failure: A randomized clinical trial. JAMA 2015, 313, 379–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, J.; Ramelet, A.S.; van Dijk, M.; Pokorna, P.; Wielenga, J.; Tume, L.; Tibboel, D.; Ista, E. Clinical recommendations for pain, sedation, withdrawal and delirium assessment in critically ill infants and children: An ESPNIC position statement for healthcare professionals. Intensive Care Med. 2016, 42, 972–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simone, S.; Edwards, S.; Lardieri, A.; Walker, L.K.; Graciano, A.L.; Kishk, O.A.; Custer, J.W. Implementation of an ICU Bundle: An Interprofessional Quality Improvement Project to Enhance Delirium Management and Monitor Delirium Prevalence in a Single PICU. Pediatr. Crit. Care Med. 2017, 18, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Herrup, E.A.; Wieczorek, B.; Kudchadkar, S.R. Feasibility and Perceptions of PICU Diaries. Pediatr. Crit. Care Med. 2019, 20, e83–e90. [Google Scholar] [CrossRef]
- Lynch, F.; Endacott, R.; Latour, J.M. Patient diaries: Survey of paediatric intensive care units in the United Kingdom and Ireland. Nurs. Crit. Care 2020, 25, 31–36. [Google Scholar] [CrossRef] [Green Version]
- McIlroy, P.A.; King, R.S.; Garrouste-Orgeas, M.; Tabah, A.; Ramanan, M. The Effect of ICU Diaries on Psychological Outcomes and Quality of Life of Survivors of Critical Illness and Their Relatives: A Systematic Review and Meta-Analysis. Crit. Care Med. 2019, 47, 273–279. [Google Scholar] [CrossRef]
- Samuel, V.M.; Colville, G.A.; Goodwin, S.; Ryninks, K.; Dean, S. The Value of Screening Parents for Their Risk of Developing Psychological Symptoms After PICU: A Feasibility Study Evaluating a Pediatric Intensive Care Follow-Up Clinic. Pediatr. Crit. Care Med. 2015, 16, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.N.; Kirby, A.; Piantino, J. If You Build It, They Will Come: Initial Experience with a Multi-Disciplinary Pediatric Neurocritical Care Follow-Up Clinic. Children 2017, 4, 83. [Google Scholar] [CrossRef] [Green Version]
- Colville, G.A.; Cream, P.R.; Kerry, S.M. Do parents benefit from the offer of a follow-up appointment after their child’s admission to intensive care?: An exploratory randomised controlled trial. Intensive Crit. Care Nurs. 2010, 26, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Manning, J.C.; Scholefield, B.R.; Popejoy, E.; Dodds, E.; Latour, J.M. Paediatric intensive care follow-up provision in the United Kingdom and Republic of Ireland. Nurs. Crit. Care 2021, 26, 128–134. [Google Scholar] [CrossRef]
- Choong, K. Early Rehabilitation in Critically Ill Children-The PICU Liber8 Study (PICULiber8); NCT03573479; U.S. National Library of Medicine: Bethesda, MD, USA, 2019. Available online: www.clinicaltrials.gov (accessed on 2 February 2021).
- Blackwood, B.; Agus, A.; Boyle, R.; Clarke, M.; Hemming, K.; Jordan, J.; Macrae, D.; McAuley, D.F.; McDowell, C.; McIlmurray, L.; et al. Sedation AND Weaning In Children (SANDWICH): Protocol for a cluster randomised stepped wedge trial. BMJ Open 2019, 9, e031630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudchadkar, S.R. PICU Up! A Pilot Stepped-Wedge Trial of a Multicomponent Early Mobility Intervention for Critically Ill Children (PICU Up!); NCT03860168; U.S. National Library of Medicine: Bethesda, MD, USA, 2019. Available online: www.clinicaltrials.gov (accessed on 2 February 2021).
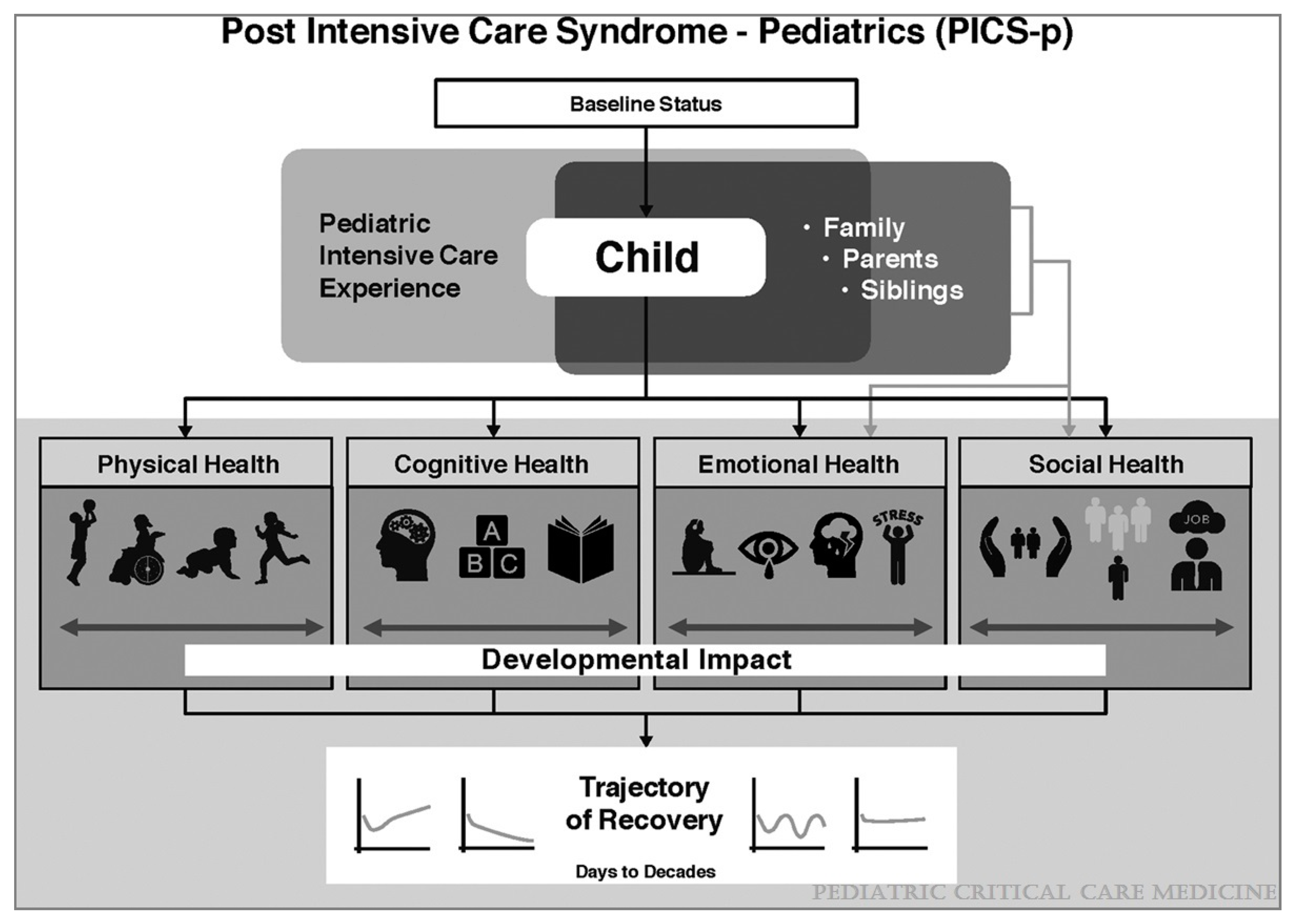
| PICs-P Domains | Examples | ||
|---|---|---|---|
| Physical Health | Chronic organ dysfunction or failure Technology dependence Chronic pain Feeding problems or malnutrition Fatigue or weakness Sleep disturbances. |  | Health-Related Quality of Life |
| Cognitive Health | Reduced attention Memory problems Decreased communication abilities Decreased school achievement. | ||
| Emotional Health | Depression Post-traumatic stress symptoms and disorder Anxiety Delusional memories and fears Behavioral problems Sleep disturbances. | ||
| Social Health | Loss of peer relationships Loss of social identity School absenteeism Decreased participation Strained family relationships Social anxiety. | ||
| PICs-F (Family) | Parent, caretaker or sibling psychiatric complications Job loss Food or housing insecurity Strained family relationships Family financial strain | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woodruff, A.G.; Choong, K. Long-Term Outcomes and the Post-Intensive Care Syndrome in Critically Ill Children: A North American Perspective. Children 2021, 8, 254. https://0-doi-org.brum.beds.ac.uk/10.3390/children8040254
Woodruff AG, Choong K. Long-Term Outcomes and the Post-Intensive Care Syndrome in Critically Ill Children: A North American Perspective. Children. 2021; 8(4):254. https://0-doi-org.brum.beds.ac.uk/10.3390/children8040254
Chicago/Turabian StyleWoodruff, Alan G., and Karen Choong. 2021. "Long-Term Outcomes and the Post-Intensive Care Syndrome in Critically Ill Children: A North American Perspective" Children 8, no. 4: 254. https://0-doi-org.brum.beds.ac.uk/10.3390/children8040254






