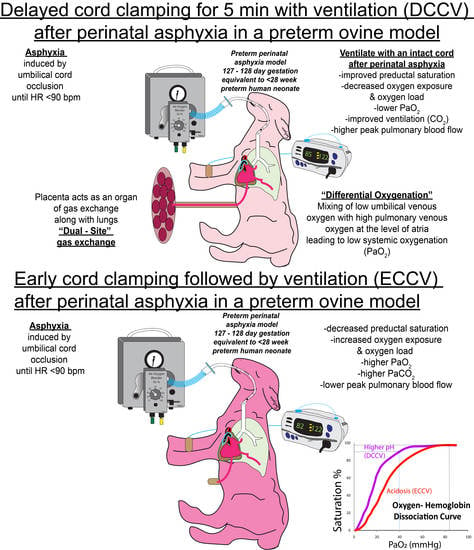
Resuscitation with an Intact Cord Enhances Pulmonary Vasodilation and Ventilation with Reduction in Systemic Oxygen Exposure and Oxygen Load in an Asphyxiated Preterm Ovine Model
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Oxygenation
3.2. Ventilation
3.3. Hemodynamics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- American Academy of Pediatrics. Textbook of Neonatal Resuscitation (NRP), 7th ed.; American Academy of Pediatrics: Elk Grove Village, IL, USA, 2016; p. 326. [Google Scholar]
- Wyckoff, M.H.; Wyllie, J.; Aziz, K.; de Almeida, M.F.; Fabres, J.; Fawke, J.; Guinsburg, R.; Hosono, S.; Isayama, T.; Kapadia, V.S.; et al. Neonatal Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 2020, 142, S185–S221. [Google Scholar] [CrossRef]
- Wyckoff, M.H.; Wyllie, J.; Aziz, K.; de Almeida, M.F.; Fabres, J.W.; Fawke, J.; Guinsburg, R.; Hosono, S.; Isayama, T.; Kapadia, V.S.; et al. Neonatal Life Support 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Resuscitation 2020, 156, A156–A187. [Google Scholar] [CrossRef]
- Welsford, M.; Nishiyama, C.; Shortt, C.; Weiner, G.; Roehr, C.C.; Isayama, T.; Dawson, J.A.; Wyckoff, M.H.; Rabi, Y. International Liaison Committee on Resuscitation Neonatal Life Support Task, F. Initial Oxygen Use for Preterm Newborn Resuscitation: A Systematic Review With Meta-analysis. Pediatrics 2019, 143. [Google Scholar] [CrossRef] [Green Version]
- Oei, J.L.; Saugstad, O.D.; Lui, K.; Wright, I.M.; Smyth, J.P.; Craven, P.; Wang, Y.A.; McMullan, R.; Coates, E.; Ward, M.; et al. Targeted Oxygen in the Resuscitation of Preterm Infants, a Randomized Clinical Trial. Pediatrics 2017, 139, e20161452. [Google Scholar] [CrossRef] [Green Version]
- Chandrasekharan, P.; Rawat, M.; Gugino, S.F.; Koenigsknecht, C.; Helman, J.; Nair, J.; Vali, P.; Lakshminrusimha, S. Effect of various inspired oxygen concentrations on pulmonary and systemic hemodynamics and oxygenation during resuscitation in a transitioning preterm model. Pediatr. Res. 2018, 84, 743–750. [Google Scholar] [CrossRef]
- March of Dimes. Available online: https://www.marchofdimes.org/complications/premature-babies.aspx (accessed on 1 November 2020).
- Chiruvolu, A.; George, R.; Stanzo, K.C.; Kindla, C.M.; Desai, S. Effects of Placental Transfusion on Late Preterm Infants Admitted to a Mother Baby Unit. Am. J. Perinatol. 2021. [Google Scholar] [CrossRef]
- Fogarty, M.; Osborn, D.A.; Askie, L.; Seidler, A.L.; Hunter, K.; Lui, K.; Simes, J.; Tarnow-Mordi, W. Delayed vs early umbilical cord clamping for preterm infants: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2018, 218, 1–18. [Google Scholar] [CrossRef]
- Katheria, A.; Poeltler, D.; Durham, J.; Steen, J.; Rich, W.; Arnell, K.; Maldonado, M.; Cousins, L.; Finer, N. Neonatal Resuscitation with an Intact Cord: A Randomized Clinical Trial. J. Pediatr. 2016, 178, 75–80.e73. [Google Scholar] [CrossRef] [Green Version]
- Knol, R.; Brouwer, E.; van den Akker, T.; DeKoninck, P.; van Geloven, N.; Polglase, G.R.; Lopriore, E.; Herkert, E.; Reiss, I.K.M.; Hooper, S.B.; et al. Physiological-based cord clamping in very preterm infant -Randomised controlled trial on effectiveness of stabilisation. Resuscitation 2020, 147, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Mercer, J.S.; McGrath, M.M.; Hensman, A.; Silver, H.; Oh, W. Immediate and delayed cord clamping in infants born between 24 and 32 weeks: A pilot randomized controlled trial. J. Perinatol. 2003, 23, 466–472. [Google Scholar] [CrossRef] [Green Version]
- Padilla-Sanchez, C.; Baixauli-Alacreu, S.; Canada-Martinez, A.J.; Solaz-Garcia, A.; Alemany-Anchel, M.J.; Vento, M. Delayed vs Immediate Cord Clamping Changes Oxygen Saturation and Heart Rate Patterns in the First Minutes after Birth. J. Pediatr. 2020, 227, 149–156.e141. [Google Scholar] [CrossRef]
- Perlman, J.M.; Wyllie, J.; Kattwinkel, J.; Wyckoff, M.H.; Aziz, K.; Guinsburg, R.; Kim, H.S.; Liley, H.G.; Mildenhall, L.; Simon, W.M.; et al. Part 7: Neonatal Resuscitation: 2015 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation 2015, 132, S204–S241. [Google Scholar] [CrossRef] [Green Version]
- Pratesi, S.; Montano, S.; Ghirardello, S.; Mosca, F.; Boni, L.; Tofani, L.; Dani, C. Placental Circulation Intact Trial (PCI-T)-Resuscitation with the Placental Circulation Intact vs. Cord Milking for Very Preterm Infants: A Feasibility Study. Front. Pediatr. 2018, 6, 364. [Google Scholar] [CrossRef]
- Andersson, O.; Rana, N.; Ewald, U.; Malqvist, M.; Stripple, G.; Basnet, O.; Subedi, K.; Kc, A. Intact cord resuscitation versus early cord clamping in the treatment of depressed newborn infants during the first 10 minutes of birth (Nepcord III)—A randomized clinical trial. Matern Health Neonatol. Perinatol. 2019, 5, 15. [Google Scholar] [CrossRef]
- Armanian, A.M.; Badiee, Z. Resuscitation of preterm newborns with low concentration oxygen versus high concentration oxygen. J. Res. Pharm. Pract. 2012, 1, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Bancalari, A.; Araneda, H.; Echeverria, P.; Marinovic, A.; Manriquez, C. Arterial oxygen saturation and heart rate in term newborn in the first hour after birth. Rev. Chil. Pediatr. 2019, 90, 384–391. [Google Scholar] [CrossRef]
- Bhatt, S.; Alison, B.J.; Wallace, E.M.; Crossley, K.J.; Gill, A.W.; Kluckow, M.; te Pas, A.B.; Morley, C.J.; Polglase, G.R.; Hooper, S.B. Delaying cord clamping until ventilation onset improves cardiovascular function at birth in preterm lambs. J. Physiol. 2013, 591, 2113–2126. [Google Scholar] [CrossRef] [Green Version]
- Brouwer, E.; Knol, R.; Vernooij, A.S.N.; van den Akker, T.; Vlasman, P.E.; Klumper, F.; DeKoninck, P.; Polglase, G.R.; Hooper, S.B.; Te Pas, A.B. Physiological-based cord clamping in preterm infants using a new purpose-built resuscitation table: A feasibility study. Arch. Dis. Child Fetal. Neonatal. Ed. 2019, 104, F396–F402. [Google Scholar] [CrossRef] [Green Version]
- Hutchon, D.J. Ventilation before Umbilical Cord Clamping Improves Physiological Transition at Birth or “Umbilical Cord Clamping before Ventilation is Established Destabilizes Physiological Transition at Birth”. Front. Pediatr. 2015, 3, 29. [Google Scholar] [CrossRef] [Green Version]
- Kc, A.; Singhal, N.; Gautam, J.; Rana, N.; Andersson, O. Effect of early versus delayed cord clamping in neonate on heart rate, breathing and oxygen saturation during first 10 minutes of birth-randomized clinical trial. Matern Health Neonatol. Perinatol. 2019, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Polglase, G.R.; Blank, D.A.; Barton, S.K.; Miller, S.L.; Stojanovska, V.; Kluckow, M.; Gill, A.W.; LaRosa, D.; Te Pas, A.B.; Hooper, S.B. Physiologically based cord clamping stabilises cardiac output and reduces cerebrovascular injury in asphyxiated near-term lambs. Arch. Dis. Child Fetal. Neonatal. Ed. 2018, 103, F530–F538. [Google Scholar] [CrossRef]
- Polglase, G.R.; Dawson, J.A.; Kluckow, M.; Gill, A.W.; Davis, P.G.; Te Pas, A.B.; Crossley, K.J.; McDougall, A.; Wallace, E.M.; Hooper, S.B. Ventilation onset prior to umbilical cord clamping (physiological-based cord clamping) improves systemic and cerebral oxygenation in preterm lambs. PLoS ONE 2015, 10, e0117504. [Google Scholar] [CrossRef]
- Lorente-Pozo, S.; Parra-Llorca, A.; Nunez-Ramiro, A.; Cernada, M.; Hervas, D.; Boronat, N.; Sandoval, J.; Vento, M. The Oxygen Load Supplied during Delivery Room Stabilization of Preterm Infants Modifies the DNA Methylation Profile. J. Pediatr. 2018. [Google Scholar] [CrossRef]
- Chandrasekharan, P.; Rawat, M.; Lakshminrusimha, S. How Do We Monitor Oxygenation during the Management of PPHN? Alveolar, Arterial, Mixed Venous Oxygen Tension or Peripheral Saturation? Children 2020, 7, 180. [Google Scholar] [CrossRef]
- Kapadia, V.S.; Chalak, L.F.; DuPont, T.L.; Rollins, N.K.; Brion, L.P.; Wyckoff, M.H. Perinatal asphyxia with hyperoxemia within the first hour of life is associated with moderate to severe hypoxic-ischemic encephalopathy. J. Pediatr. 2013, 163, 949–954. [Google Scholar] [CrossRef]
- Noori, S.; McCoy, M.; Anderson, M.P.; Ramji, F.; Seri, I. Changes in cardiac function and cerebral blood flow in relation to peri/intraventricular hemorrhage in extremely preterm infants. J. Pediatr. 2014, 164, 264–270.e3. [Google Scholar] [CrossRef] [PubMed]
- Noori, S.; Seri, I. Hemodynamic antecedents of peri/intraventricular hemorrhage in very preterm neonates. Semin. Fetal. Neonatal. Med. 2015, 20, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Lakshminrusimha, S.; Russell, J.A.; Steinhorn, R.H.; Swartz, D.D.; Ryan, R.M.; Gugino, S.F.; Wynn, K.A.; Kumar, V.H.; Mathew, B.; Kirmani, K.; et al. Pulmonary hemodynamics in neonatal lambs resuscitated with 21%, 50%, and 100% oxygen. Pediatr. Res. 2007, 62, 313–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munkeby, B.H.; Borke, W.B.; Bjornland, K.; Sikkeland, L.I.; Borge, G.I.; Lomo, J.; Rivera, S.; Khrestchatisky, M.; Halvorsen, B.; Saugstad, O.D. Resuscitation of hypoxic piglets with 100% O2 increases pulmonary metalloproteinases and IL-8. Pediatr. Res. 2005, 58, 542–548. [Google Scholar] [CrossRef] [Green Version]
- Wedgwood, S.; Steinhorn, R.H.; Lakshminrusimha, S. Optimal oxygenation and role of free radicals in PPHN. Free Radic. Biol. Med. 2019, 142, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Borke, W.B.; Munkeby, B.H.; Halvorsen, B.; Bjornland, K.; Tunheim, S.H.; Borge, G.I.; Thaulow, E.; Saugstad, O.D. Increased myocardial matrix metalloproteinases in hypoxic newborn pigs during resuscitation: Effects of oxygen and carbon dioxide. Eur. J. Clin. Investig. 2004, 34, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Markus, T.; Hansson, S.; Amer-Wahlin, I.; Hellstrom-Westas, L.; Saugstad, O.D.; Ley, D. Cerebral inflammatory response after fetal asphyxia and hyperoxic resuscitation in newborn sheep. Pediatr. Res. 2007, 62, 71–77. [Google Scholar] [CrossRef] [Green Version]
- Hitchler, M.J.; Domann, F.E. An epigenetic perspective on the free radical theory of development. Free Radic. Biol. Med. 2007, 43, 1023–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrasekharan, P.; Lakshminrusimha, S. Oxygen therapy in preterm infants with pulmonary hypertension. Semin. Fetal. Neonatal. Med. 2020, 25, 101070. [Google Scholar] [CrossRef]
- Custer, J.R.; Hales, C.A. Influence of alveolar oxygen on pulmonary vasoconstriction in newborn lambs versus sheep. Am. Rev. Respir. Dis. 1985, 132, 326–331. [Google Scholar] [CrossRef] [PubMed]








| Characteristics | ECC + V (N = 8) | DCC + V (N = 7) |
|---|---|---|
| Gestational age (days) | 127 ± 0.52 | 128 ± 0.84 |
| Female (N) | 4 | 3 |
| Birth weight (kg) | 3.3 ± 0.63 | 3.3 ± 0.70 |
| Born by multiplicity (N) | Twin–6 | Twin–4 |
| Heart rate at asphyxia (bpm) | 88 ± 8 | 86 ± 10 |
| Mean blood pressure at asphyxia (mmHg) | 36 ± 8 | 34 ± 10 |
| pH before resuscitation | 7.04 ± 0.08 | 7.0 ± 0.08 |
| PaCO2 before resuscitation( mmHg) | 90 ± 25 | 101 ± 23 |
| PaO2 before resuscitation (mmHg) | 14 ± 6 | 15 ± 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandrasekharan, P.; Gugino, S.; Helman, J.; Koenigsknecht, C.; Nielsen, L.; Bradley, N.; Nair, J.; Agrawal, V.; Bawa, M.; Mari, A.; et al. Resuscitation with an Intact Cord Enhances Pulmonary Vasodilation and Ventilation with Reduction in Systemic Oxygen Exposure and Oxygen Load in an Asphyxiated Preterm Ovine Model. Children 2021, 8, 307. https://0-doi-org.brum.beds.ac.uk/10.3390/children8040307
Chandrasekharan P, Gugino S, Helman J, Koenigsknecht C, Nielsen L, Bradley N, Nair J, Agrawal V, Bawa M, Mari A, et al. Resuscitation with an Intact Cord Enhances Pulmonary Vasodilation and Ventilation with Reduction in Systemic Oxygen Exposure and Oxygen Load in an Asphyxiated Preterm Ovine Model. Children. 2021; 8(4):307. https://0-doi-org.brum.beds.ac.uk/10.3390/children8040307
Chicago/Turabian StyleChandrasekharan, Praveen, Sylvia Gugino, Justin Helman, Carmon Koenigsknecht, Lori Nielsen, Nicole Bradley, Jayasree Nair, Vikash Agrawal, Mausma Bawa, Andreina Mari, and et al. 2021. "Resuscitation with an Intact Cord Enhances Pulmonary Vasodilation and Ventilation with Reduction in Systemic Oxygen Exposure and Oxygen Load in an Asphyxiated Preterm Ovine Model" Children 8, no. 4: 307. https://0-doi-org.brum.beds.ac.uk/10.3390/children8040307