Characterization of Speech and Language Phenotype in GLUT1DS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Materials and Procedures
2.3. Statistical Analysis
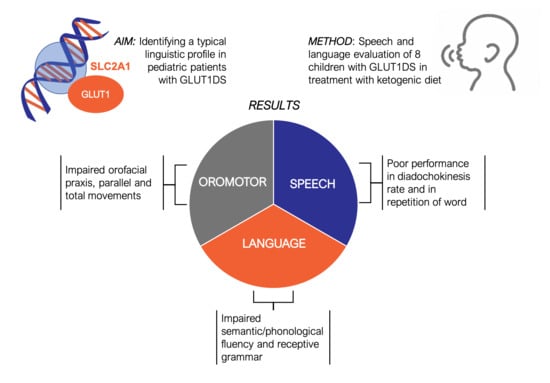
3. Results
3.1. Tests Evaluation
3.1.1. Oromotor Skills
3.1.2. Speech
3.1.3. Language
3.1.4. Intelligence Quotient
3.2. Correlations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Daci, A.; Bozalija, A.; Jashari, F.; Krasniqi, S. Individualizing Treatment Approaches for Epileptic Patients with Glucose Transporter Type1 (GLUT-1) Deficiency. Int. J. Mol. Sci. 2018, 19, 122. [Google Scholar] [CrossRef] [Green Version]
- Klepper, J.; Akman, C.; Armeno, M.; Auvin, S.; Cervenka, M.; Cross, H.J.; De Giorgis, V.; Della Marina, A.; Engelstad, K.; Heussinger, N.; et al. Glut1 Deficiency Syndrome (Glut1DS): State of the art in 2020 and recommendations of the international Glut1DS study group. Epilepsia Open 2020. [Google Scholar] [CrossRef] [PubMed]
- Alter, A.S.; Engelstad, K.; Hinton, V.J.; Montes, J.; Pearson, T.S.; Akman, C.I.; De Vivo, D.C. Long-term clinical course of Glut1 deficiency syndrome. J. Child. Neurol. 2015, 30, 160–169. [Google Scholar] [CrossRef] [PubMed]
- De Giorgis, V.; Masnada, S.; Varesio, C.; Chiappedi, M.A.; Zanaboni, M.; Pasca, L.; Filippini, M.; Macasaet, J.A.; Valente, M.; Ferraris, C.; et al. Overall cognitive profiles in patients with GLUT1 Deficiency Syndrome. Brain Behav. 2019, 9, 01224. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Takahashi, S.; Kagitani-Shimono, K.; Natsume, J.; Yanagihara, K.; Fujii, T.; Oguni, H. Nationwide survey of glucose transporter-1 deficiency syndrome (GLUT-1DS) in Japan. Brain Dev. 2015, 37, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Hully, M.; Vuillaumier-Barrot, S.; Le Bizec, C.; Boddaert, N.; Kaminska, A.; Lascelles, K.; de Lonlay, P.; Cances, C.; Portes, V.D.; Roubertie, A.; et al. From splitting GLUT1 deficiency syndromes to overlapping phenotypes. Eur. J. Med. Genet. 2015, 58, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.; Johannesen, K.M.; Ek, J.; Tang, S.; Marini, C.; Blichfeldt, S.; Kibaek, M.; Von Spiczak, S.; Weckhuysen, S.; Frangu, M.; et al. The role of SLC2A1 mutations in myoclonic astatic epilepsy and absence epilepsy, and the estimated frequency of GLUT1 deficiency syndrome. Epilepsia 2015, 56, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Leen, W.G.; Klepper, J.; Verbeek, M.M.; Leferink, M.; Hofste, T.; Willemsen, M.A. Glucose transporter-1 deficiency syndrome: The expanding clinical and genetic spectrum of a treatable disorder. Brain 2010, 133, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Pearson, T.S.; Akman, C.; Hinton, V.J.; Engelstad, K.; De Vivo, D.C. Phenotypic spectrum of glucose transporter type 1 deficiency syndrome (Glut1 DS). Curr. Neurol. Neurosci. Rep. 2013, 13, 342. [Google Scholar] [CrossRef] [PubMed]
- Ramm-Pettersen, A.; Stabell, K.E.; Nakken, K.O.; Selmer, K.K. Does ketogenic diet improve cognitive function in patients with GLUT1-DS? A 6- to 17-month follow-up study. Epilepsy Behav. 2014, 39, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Chugani, H.T.; Phelps, M.E. Maturational changes in cerebral function in infants determined by 18FDG positron emission tomography. Science 1986, 231, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Karmiloff-Smith, A. Development itself is the key to understanding developmental disorders. Trends Cogn. Sci. 1998, 2, 389–398. [Google Scholar] [CrossRef]
- Bearzotti, F.; Fabbro, F. Test per la valutazione delle Prassie orofacciali nel bambino, Protocollo B e C (in Italian). G. di Neuropsichiatria dell’Età Evol. 2003, 23, 406–417. [Google Scholar]
- Fanzago, F. Test di Valutazione Dell’articolazione (in Italian); Centro Stampa Palazzo Maldura: Padova, Italy, 1986. [Google Scholar]
- Cossu, G. TNL—Test Neuropsicologico Lessicale Per l’età Evolutiva (in Italian); Hogrefe: Firenze, Italy, 2013. [Google Scholar]
- Chilosi, A.M.; Lorenzini, I.; Fiori, S.; Graziosi, V.; Rossi, G.; Pasquariello, R.; Cipriani, P.; Cion, G. Behavioral and neurobiological correlates of childhood apraxia of speech in Italian children. Brain Lang. 2015, 150, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Dunn, L.M.; Dunn, L.M. Peabody Picture Vocabulary Test—Revised. Minneapolis: NCS Pearson; 2007 Standardised for Italian Children; Stella, G., Pizzoli, P., Tressoldi, P.E., Eds.; Omega: Torino, Italy, 2000. [Google Scholar]
- Bisiacchi, P.S.; Cendron, M.; Gugliotta, M.; Tressoldi, P.E.; Vio, C. BVN 5-11 Batteria di Valutazione Neuropsicologica per l’età Evolutiva (in Italian); Erickson: Trento, Italy, 2005. [Google Scholar]
- Korkman, M.; Kirk, U.; Kemp, S. NEPSY-II: Clinical and interpretative manual. San Antonio TX: Harcourt Assessment; 2007. Standardised for Italian Children; Urgesi, C., Campanella, F., Fabbro, F., Eds.; Giunti OS: Firenze, Italy, 2011. [Google Scholar]
- Wechsler, D. WISC-IV: Wechsler Intelligence Scale for Children—Fourth Edition; Psychological Corporation: San Antonio, TX, USA, 2003. [Google Scholar]
- Wechsler, D. WPSSI III: Wechsler Preschool and Primary Scale of Intelligence; Psychological Corporation: New York, NY, USA, 1967. [Google Scholar]
- Klepper, J.; Scheffer, H.; Leiendecker, B.; Gertsen, E.; Binder, S.; Leferink, M.; Hertzberg, C.; Näke, A.; Voit, T.; Willemsen, M.A. Seizure control and acceptance of the ketogenic diet in GLUT1 deficiency syndrome: A 2- to 5-year follow-up of 15 children enrolled prospectively. Neuropediatrics 2005, 36, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Pascual, J.M.; Yang, H.; Engelstad, K.; Jhung, S.; Sun, R.P.; De Vivo, D.C. Glut-1 deficiency syndrome: Clinical, genetic, and therapeutic aspects. Ann. Neurol. 2005, 57, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Oller, D.K.; Eilers, R.E.; Neal, A.R.; Schwartz, H.K. Precursors to speech in infancy: The prediction of speech and language disorders. J. Commun. Disord. 1999, 32, 223–245. [Google Scholar] [CrossRef]
- Vihman, M.M. Applied language studies. In Phonological Development: The Origins of Language in the Child; Blackwell Publishing: Oxford, UK, 1996. [Google Scholar]
- Westermann, G.; Reck Miranda, E. A new model of sensorimotor coupling in the development of speech. Brain Lang. 2004, 89, 393–400. [Google Scholar] [CrossRef]
- Shriberg, L.D.; Aram, D.M.; Kwiatkowski, J. Developmental apraxia of speech: III. A subtype marked by inappropriate stress. J. Speech Lang. Hear. Res. 1997, 40, 313–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Pt1 | Pt2 | Pt3 | Pt4 | Pt5 | Pt6 | Pt7 | Pt8 | All (n = 8) | |
|---|---|---|---|---|---|---|---|---|---|
| Age at evaluation (years) | 6.1 | 13.9 | 15.4 | 4.6 | 10.3 | 11.2 | 11.4 | 7.11 | M 10 (SD 3.79) range 4.6–15.4 |
| Age at GLUT1DS Diagnosis (months) | 59 | 60 | 75 | 24 | 122 | 79 | 95 | 69 | M 72.87 (SD 26.70) range 24–122 |
| CSF/serum glucose ratio | 0.37 | NA | 0.34 | 0.27 | NA | 0.35 | 0.43 | 0.37 | - |
| Genotype | DeletionExones1–10 | Missense R400C | Deletion 1p34.2 | Truncatin Y366X | Missense G314S | Missense N34S | Missense G17R | Frameshift L486fs | - |
| GLUT1DS Family history | no | no | no | no | yes | yes | no | no | yes 25% |
| Perinatal Period | un | un | un | un | un | un | un | Prematurity jaundice | yes 12.5% |
| Recurrent otitis | no | yes | no | no | no | no | no | no | yes 12.5% |
| Auditory deficits | no | no | no | no | no | no | no | no | yes 0% |
| Babbling onset (months) | 18 | 12 | 24 | 10 | 9 | 10 | 10 | na | M 13.29 (SD 5.61) range 9–24 |
| Age at first word (months) | 30 | 24 | 24 | 20 | 12 | 12 | 12 | 12 | M 18.25 (SD 7.2) range 12–30 |
| Age at combinatory speech (months) | 60 | 24 | 60 | 24 | 24 | 24 | 24 | 36 | M 34.5 (SD 16.27) range 24–60 |
| Preschool speech intelligibility | no | yes | no | no | yes | yes | yes | yes | no 37.5% |
| Language disorder | dysarthria | speech | dysarthria | speech | speech | none | none | none | speech 37.5% dysarthria 25% |
| Psychomoto development | delayed | normal | delayed | normal | normal | normal | normal | normal | delayed 25% |
| Intellectual disability | borderline | borderline | moderate | normal | borderline | borderline | mild | borderline | - |
| Epilepsy Family history | yes | no | yes | no | yes | yes | no | no | yes 50% |
| Epilepsy | yes | yes | yes | no | yes | yes | yes | yes | yes 87.5% |
| Epilepsy onset (months) | 24 | 36 | 26 | na | 84 | 60 | 12 | 12 | M 36.28 (SD24.71) range 12–84 |
| Movement disorder | yes | yes | yes | yes | yes | yes | yes | yes | yes 100% |
| Movement disorder severity | moderate | mild | mild | mild | mild | mild | mild | mild | mild 87.5% moderate 12.5% |
| Age at KDTs Initiation (months) | 60 | 60 | 98 | 24 | 121 | 88 | 94 | 69 | M 76.75 (SD 29.85) range 24–121 |
| Rehabilitation therapy | speech | psychomotor & speech | speech | psychomotor & speech | speech & cognitive | psychomotor & speech | none | psychomotor | speech 75% psychomotor 50% cognitive 12.5% |
| Rehabilitation onset (months) | 24 | 60 | 24 | 24 | 108 | 60 | na | 48 | M 49.71 (SD 30.53) range 24–108 |
| Rehabilitation’s duration (years) | 6 | 6 | 8 | 3 | 1.5 | 7 | na | 2 | M 4.78 (SD 2.57) range 1.5–8 |
| Task | Description |
|---|---|
| Parental report on clinical history | Child’s pre-peri and post-natal clinically events and speech and language milestones acquisition. |
| Oromotor skills | Oromotor skills were examined with Orofacial Praxis [13]. Oromotor skills were the ability to plan and execute movements or sequences of voluntary movements, meaningful or not, using the muscles of the pharyngo-buccofacial system or the orofacial region. The Orofacial Praxis Test, consisting of 36 gestures, 24 single and 12 complex, elicited through verbal and imitative request. |
| Phonetic inventory | Phonetic inventory was investigated with the Articulation Test of Fanzago [14].This instrument was based on spontaneous/repetition elicited denomination of 114 figures which named allow to verify whether the target phoneme (place in different positions within the word) has been produced correctly or replaced/omitted/distorted. |
| Phonological Planning | Phonological Planning was tested by the Repetition of 31 words pronounced by the examiner [15]. This subtest is designed to assess phonological encoding and decoding through the repetition of words and it allows to detect the presence of phonological processes. For each word it is possible to calculate the number and the type of phonological processes produced. It is possible to identify two phonological processes, simplification and atypical. Simplification processes represent the persistence of normal primitive processes in successive stages of phonological development. Atypical idiosyncratic processes included types of simplifications rarely found in normal language development, or those that that are never found in normal developmental processes. |
| Diadochokinesis | Diadochokinesis was assessed with Maximum performance rate [16]. This task is used to test the ability to repeat a syllable sequence (/pataka/) as quickly as possible for 20 s in order to look at motor speech skills separate from the effects related to word familiarity. |
| Receptive vocabulary | Receptive vocabulary was evaluated by PPVT-III [17]. The PPVT is a receptive vocabulary test in which the child points to one of four pictures on a page that is named by the examiner. |
| Expressive vocabulary | Expressive vocabulary was tested by the Name BVN 5-11 [18]. For this task, the subject is asked to name 20 (for children aged from 5 to 11) or 88 (for children aged from 12 to 18) figures in order to measure patient’s vocabulary ability. |
| Receptive grammar | Receptive grammar was examined with Comprehension of Instructions NEPSY [19]. This task assesses receptive language and it involves understanding verbal instructions and processing them into actions. |
| Expressive grammar | Expressive grammar was evaluated by Sentence repetition NEPSY II [19]. This task was used to investigate the production of grammar structures. |
| Verbal Fluency | Verbal Fluency was examined with Word generation NEPSY II [19]. This subtest is designed to assess verbal productivity through the ability to generate words and it consists of two tasks: semantic or phonemic fluency. The participants are given 1 min to generate as many words as possible within a semantic category or they are asked to say words that start with a given letter. |
| Cognitive assessment | Age-appropriate versions of the Wechsler scales were administered to assess intellectual ability: —from 6 to 16 years the Wechsler Intelligence Scale for Children—Fourth Edition [20] or 2–6 year olds, the Wechsler Preschool and Primary Scale of Intelligence—Third Edition, Italian version (WPPSI-III) [21] and –Full-scale IQ (FSIQ) scores were derived and classified according to test manual normative data. |
| Pt1 | Pt2 | Pt3 | Pt4 | Pt5 | Pt6 | Pt7 | Pt8 | All (n = 8) | |
|---|---|---|---|---|---|---|---|---|---|
| Voiced praxis verbal request (zs) | −2 | 1.37 | −2 | −1.70 | −3.68 | −1.16 | 1.37 | −0.3 | M −1.01 (SD 1.74) range −3.68 1.37 |
| Voiced praxis imitation (zs) | −3.22 | 0.54 | 0.54 | −1.17 | −5.11 | −1.33 | 0.54 | −3.22 | M −1.55 (SD 2.12) range −5.11 0.54 |
| Orofacial praxis verbal request (zs) | −1.33 | −3.41 | −1.33 | −2.11 | −9.66 | −3.41 | 0.75 | −1.33 | M −2.72 (SD 3.1) range −9.66 0.75 |
| Orofacial praxis imitation (zs) | −1.90 | 0.53 | −1.73 | 0.71 | −1.90 | −1.90 | 0.53 | 0.53 | M −0.64 (SD1.3) range −1.9 0.71 |
| Sequence movements verbal request (zs) | −2.91 | −0.5 | −2.84 | 0.38 | −0.5 | −0.5 | 0.69 | −2.91 | M −1.13 (SD 1.51) range −2.91 0.69 |
| Sequence movements imitation (zs) | −4.74 | 0.43 | −3 | −0.28 | 0.43 | 0.43 | 0.43 | −1.29 | M −0.94 (SD 1.95) range −4.74 0.43 |
| Parallel movements verbal request (zs) | −6.09 | −2.80 | 0.69 | 1.10 | −6.09 | −6.09 | −6.09 | 0.69 | M −3.08 (SD 3.42) range −6.09 1.10 |
| Parallel movements imitation (zs) | −10.31 | 0.21 | 0.21 | 0.75 | 0.21 | −5.05 | −5.05 | 0.21 | M −2.35 (SD 4.03) range −10.31 0.75 |
| Total score verbal request (zs) | −4.41 | −0.77 | −3.2 | −1.15 | −6.83 | −3.2 | 0.43 | −1.98 | M −2.63 (SD 2.29) range −6.83 0.43 |
| Total score imitation (zs) | −6.26 | 0.75 | −1.87 | 0.74 | −2.75 | −1.87 | −0.12 | −1.52 | M −1.61 (SD 2.27) range −6.26 0.75 |
| Phonetic inventory: absent phonemes (des) | d g tʃ dȝ s z ts dz r ʎ | r ʎ | z s r ʎ st ts | r ʎ | none | r | ʎ r | ts dz ʎ r | - |
| Present phonemes (consonants and vowels) (n) | 16 | 24 | 22 | 24 | 26 | 25 | 25 | 22 | M 23 (SD 3.16) range 16–26 |
| Phonological Planning (zs) | −31.5 | −10.96 | −22.38 | −9.73 | −21.61 | −9.23 | −1.29 | na | M −15.24 (SD 9.52) range −31.5 −1.29 |
| Phonological Planning (word accuracy) (n) | 0/31 | 24/31 | 20/31 | 8/31 | 18/31 | 26/31 | 29/31 | na | - |
| Phonological Planning (syllabic structure accuracy) (n) | 26/31 | 28/31 | 28/31 | 31/31 | 30/31 | 31/31 | 31/31 | na | - |
| Phonological Planning (word length accuracy) (n) | 8/31 | 24/31 | 26/31 | 15/31 | 25/31 | 30/31 | 31/31 | na | - |
| Diadochokinesis (zs) | −7.86 | −0.53 | −1.2 | −3.2 | −0.86 | −3.2 | 0.8 | na | M −0.27(SD 3.48) range −7.86 0.8 |
| Receptive vocabulary (st) | 75 | 113 | 83 | 106 | 91 | 87 | 85 | 77 | M 89.63 (SD 13.42) range 75–113 |
| Expressive vocabulary (zs) | −1.9 | 0.03 | −5.45 | −0.1 | −1 | 0 | 0.35 | 0.77 | M −0.91 (SD 2.01) range 5.45–0.77 |
| Receptive Grammar (ss) | 3 | 6 | 1 | 8 | 8 | 1 | 5 | 6 | M 4.75 (SD2.81) range 1–8 |
| Expressive Grammar (ss) | 7 | 9 | 1 | 10 | 6 | 5 | 9 | 12 | M 7.37 (SD 3.42) range 1–12 |
| Word generation: semantic fluency (ss) | 3 | 7 | 3 | 9 | 4 | 6 | 4 | 9 | M 5.62 (SD 2.5) range 3–9 |
| Word generation: phonemic fluency (ss) | na | 3 | 3 | na | 6 | 6 | 4 | na | M 4.40 (SD 1.51) range 3–6 |
| FSIQ (st) | 76 | 81 | 41 | 96 | 71 | 73 | 69 | 80 | M 73.38 (SD 15.55) range 41–96 |
| VCI (st) | 88 | 82 | 66 | 98 | 90 | 84 | 74 | 98 | M 85 (SD 11.11) range 66–98 |
| PRI (st) | 71 | 102 | 48 | 93 | 80 | 76 | 65 | 73 | M 76 (SD 16.56) range 48–102 |
| WMI (st) | na | 76 | 52 | na | 64 | 76 | 91 | na | M 71.80 (SD 14.63) range 52–91 |
| PSI (st) | 58 | 79 | 56 | na | 74 | 82 | 82 | 67 | M 71.14 (SD 10.99) range 56–82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanaboni, M.P.; Pasca, L.; Villa, B.V.; Faggio, A.; Grumi, S.; Provenzi, L.; Varesio, C.; De Giorgis, V. Characterization of Speech and Language Phenotype in GLUT1DS. Children 2021, 8, 344. https://0-doi-org.brum.beds.ac.uk/10.3390/children8050344
Zanaboni MP, Pasca L, Villa BV, Faggio A, Grumi S, Provenzi L, Varesio C, De Giorgis V. Characterization of Speech and Language Phenotype in GLUT1DS. Children. 2021; 8(5):344. https://0-doi-org.brum.beds.ac.uk/10.3390/children8050344
Chicago/Turabian StyleZanaboni, Martina Paola, Ludovica Pasca, Barbara Valeria Villa, Antonella Faggio, Serena Grumi, Livio Provenzi, Costanza Varesio, and Valentina De Giorgis. 2021. "Characterization of Speech and Language Phenotype in GLUT1DS" Children 8, no. 5: 344. https://0-doi-org.brum.beds.ac.uk/10.3390/children8050344