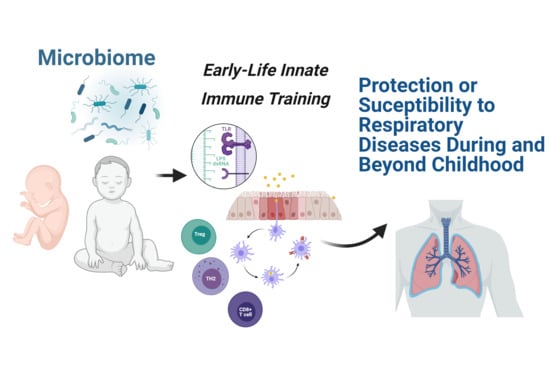
Early Microbial–Immune Interactions and Innate Immune Training of the Respiratory System during Health and Disease
Abstract
:1. Introduction
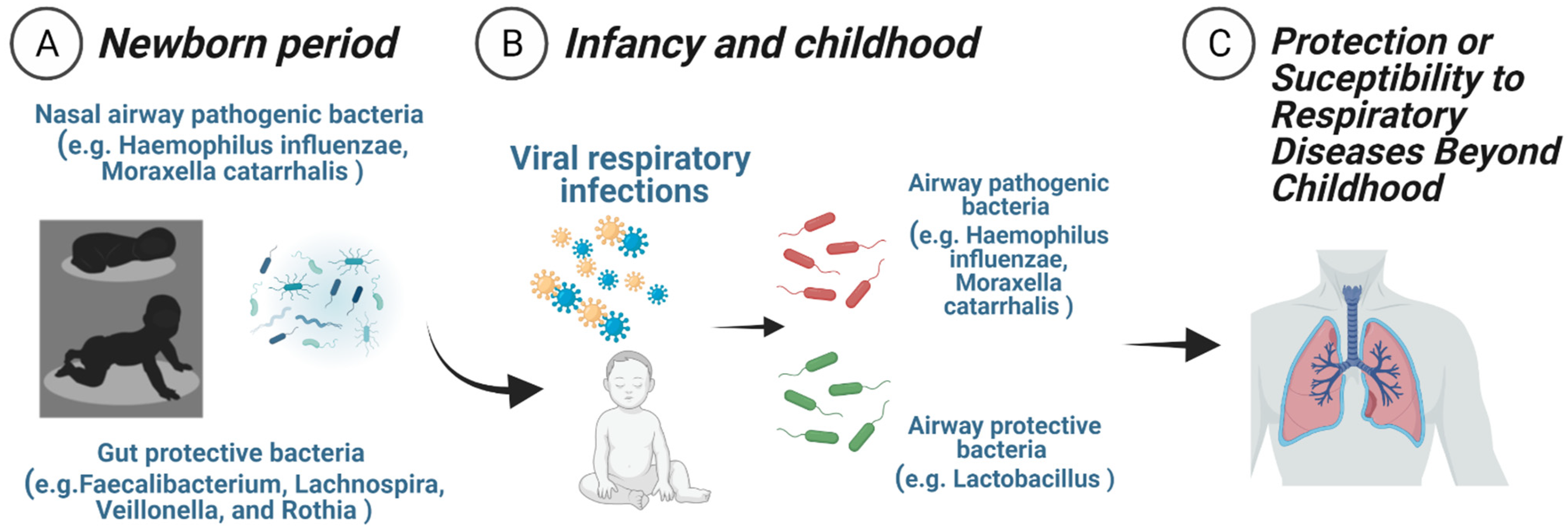
2. Neonatal Nasal Airway Microbial Populations and Respiratory Outcomes Later in Life
3. Nasal Airway Microbiota–Immune Interactions in Early Infancy
4. Neonatal Intestinal Microbial Populations and Respiratory Outcomes Later in Life
5. The Symbiosis of Bacterial Microbiota and Respiratory Viruses in Early-Life
6. Microbiome-Driven Interventions and Early Respiratory Health: The Novel Notion of Innate Immune Training
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef] [Green Version]
- Von Mutius, E.; Fritzsch, C.; Weiland, S.K.; Roll, G.; Magnussen, H. Prevalence of asthma and allergic disorders among children in united Germany: A descriptive comparison. BMJ 1992, 305, 1395–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strachan, D. Family size, infection and atopy: The first decade of the ’hygiene hypothesis’. Thorax 2000, 55, S2–S10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, M.M.; Hrusch, C.L.; Gozdz, J.; Igartua, C.; Pivniouk, V.; Murray, S.E.; Ledford, J.G.; Dos Santos, M.M.; Anderson, R.L.; Metwali, N.; et al. Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children. N. Engl. J. Med. 2016, 375, 411–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Netea, M.G.; Domínguez-Andrés, J.; Barreiro, L.B.; Chavakis, T.; Divangahi, M.; Fuchs, E.; Joosten, L.A.B.; Van Der Meer, J.W.M.; Mhlanga, M.M.; Mulder, W.J.M.; et al. Defining trained immunity and its role in health and disease. Nat. Rev. Immunol. 2020, 20, 375–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holt, P.; Strickland, D. Innate Immune Training for prevention of recurrent wheeze in early childhood. Am. J. Respir. Crit. Care Med. 2021. [Google Scholar] [CrossRef]
- Gutierrez, M.J.; Nino, G.; Hong, X.; Wang, X. Epigenomics and Early Life Human Humoral Immunity: Novel Paradigms and Research Opportunities. Front. Immunol. 2020, 11, 1766. [Google Scholar] [CrossRef]
- Lynch, S.V.; Vercelli, D. Microbiota, Epigenetics, and Trained Immunity. Convergent Drivers and Mediators of the Asthma Trajectory from Pregnancy to Childhood. Am. J. Respir. Crit. Care Med. 2021, 203, 802–808. [Google Scholar] [CrossRef]
- Martinez, F.D. Childhood Asthma Inception and Progression. Immunol. Allergy Clin. N. Am. 2019, 39, 141–150. [Google Scholar] [CrossRef]
- Martinez, F.D.; Guerra, S. Early Origins of Asthma. Role of Microbial Dysbiosis and Metabolic Dysfunction. Am. J. Respir. Crit. Care Med. 2018, 197, 573–579. [Google Scholar] [CrossRef]
- Bisgaard, H.; Hermansen, M.N.; Buchvald, F.; Loland, L.; Halkjaer, L.B.; Bønnelykke, K.; Brasholt, M.; Heltberg, A.; Vissing, N.H.; Thorsen, S.V.; et al. Childhood Asthma after Bacterial Colonization of the Airway in Neonates. N. Engl. J. Med. 2007, 357, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Vissing, N.H.; Larsen, J.M.; Rasmussen, M.A.; Chawes, B.L.K.; Thysen, A.H.; Bønnelykke, K.; Brix, S.; Bisgaard, H. Susceptibility to Lower Respiratory Infections in Childhood is Associated with Perturbation of the Cytokine Response to Pathogenic Airway Bacteria. Pediatr. Infect. Dis. J. 2016, 35, 561–566. [Google Scholar] [CrossRef] [Green Version]
- Toivonen, L.; Hasegawa, K.; Waris, M.; Ajami, N.J.; Petrosino, J.F.; Camargo, C.A., Jr.; Peltola, V. Early nasal microbiota and acute respiratory infections during the first years of life. Thorax 2019, 74, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Toivonen, L.; Karppinen, S.; Schuez-Havupalo, L.; Waris, M.; He, Q.; Hoffman, K.L.; Petrosino, J.F.; Dumas, O.; Camargo, C.A.; Hasegawa, K.; et al. Longitudinal Changes in Early Nasal Microbiota and the Risk of Childhood Asthma. Pediatrics 2020, 146, e20200421. [Google Scholar] [CrossRef] [PubMed]
- Teo, S.M.; Mok, D.; Pham, K.; Kusel, M.; Serralha, M.; Troy, N.; Holt, B.J.; Hales, B.J.; Walker, M.L.; Hollams, E.; et al. The Infant Nasopharyngeal Microbiome Impacts Severity of Lower Respiratory Infection and Risk of Asthma Development. Cell Host Microbe 2015, 17, 704–715. [Google Scholar] [CrossRef] [Green Version]
- Ta, L.D.H.; Yap, G.C.; Tay, C.J.X.; Lim, A.S.M.; Huang, C.-H.; Chu, C.W.; De Sessions, P.F.; Shek, L.P.; Goh, A.; Van Bever, H.P.; et al. Establishment of the nasal microbiota in the first 18 months of life: Correlation with early-onset rhinitis and wheezing. J. Allergy Clin. Immunol. 2018, 142, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Følsgaard, N.V.; Schjørring, S.; Chawes, B.L.K.; Rasmussen, M.A.; Krogfelt, K.A.; Brix, S.; Bisgaard, H.F. Pathogenic Bacteria Colonizing the Airways in Asymptomatic Neonates Stimulates Topical Inflammatory Mediator Release. Am. J. Respir. Crit. Care Med. 2013, 187, 589–595. [Google Scholar] [CrossRef]
- Thorsen, J.; Rasmussen, M.A.; Waage, J.; Mortensen, M.; Brejnrod, A.; Bønnelykke, K.; Chawes, B.L.; Brix, S.; Sørensen, S.J.; Stokholm, J.; et al. Infant airway microbiota and topical immune perturbations in the origins of childhood asthma. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Salka, K.; Arroyo, M.; Chorvinsky, E.; Abutaleb, K.; Perez, G.F.; Wolf, S.; Xuchen, X.; Weinstock, J.; Gutierrez, M.J.; Pérez-Losada, M.; et al. Innate IFN-lambda responses to dsRNA in the human infant airway epithelium and clinical regulatory factors during viral respiratory infections in early life. Clin. Exp. Allergy 2020, 50, 1044–1054. [Google Scholar] [CrossRef]
- Broggi, A.; Granucci, F.; Zanoni, I. Type III interferons: Balancing tissue tolerance and resistance to pathogen invasion. J. Exp. Med. 2019, 217, e20190295. [Google Scholar] [CrossRef]
- Kotenko, S.V.; Rivera, A.; Parker, D.; Durbin, J.E. Type III IFNs: Beyond antiviral protection. Semin. Immunol. 2019, 43, 101303. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Jo, A.; Jeon, Y.J.; An, S.; Lee, K.-M.; Yoon, S.S.; Choi, J.Y. Nasal commensal Staphylococcus epidermidis enhances interferon-λ-dependent immunity against influenza virus. Microbiome 2019, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Planet, P.J.; Parker, D.; Cohen, T.S.; Smith, H.; Leon, J.D.; Ryan, C.; Hammer, T.J.; Fierer, N.; Chen, E.I.; Prince, A.S. Lambda Interferon Restructures the Nasal Microbiome and Increases Susceptibility to Staphylococcus aureus Superinfection. mBio 2016, 7, e01939-15. [Google Scholar] [CrossRef] [Green Version]
- Salka, K.; Arroyo, M.; Naime, S.; Chorvinsky, E.; Gutierrez, M.J.; Pillai, D.K.; Perez, G.F.; Nino, G. TSLP Production in the Human Infant Airway Epithelium and Clinical Relevance during Viral Respiratory Infections. Am. J. Respir. Cell Mol. Biol. 2020, 62, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Stier, M.T.; Bloodworth, M.H.; Toki, S.; Newcomb, D.C.; Goleniewska, K.; Boyd, K.L.; Quitalig, M.; Hotard, A.L.; Moore, M.L.; Hartert, T.V.; et al. Respiratory syncytial virus infection activates IL-13–producing group 2 innate lymphoid cells through thymic stromal lymphopoietin. J. Allergy Clin. Immunol. 2016, 138, 814–824. [Google Scholar] [CrossRef] [Green Version]
- Nino, G.; Huseni, S.; Perez, G.F.; Pancham, K.; Mubeen, H.; Abbasi, A.; Wang, J.; Eng, S.; Colberg-Poley, A.M.; Pillai, D.K.; et al. Directional Secretory Response of Double Stranded RNA-Induced Thymic Stromal Lymphopoetin (TSLP) and CCL11/Eotaxin-1 in Human Asthmatic Airways. PLoS ONE 2014, 9, e115398. [Google Scholar] [CrossRef] [Green Version]
- Perez, G.F.; Pancham, K.; Huseni, S.; Preciado, D.; Freishtat, R.J.; Colberg-Poley, A.M.; Hoffman, E.P.; Rose, M.C.; Nino, G. Rhinovirus infection in young children is associated with elevated airway TSLP levels. Eur. Respir. J. 2014, 44, 1075–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, J.M.; Brix, S.; Thysen, A.H.; Birch, S.; Rasmussen, M.A.; Bisgaard, H. Children with asthma by school age display aberrant immune responses to pathogenic airway bacteria as infants. J. Allergy Clin. Immunol. 2014, 133, 1008–1013. [Google Scholar] [CrossRef]
- Chawes, B.L.; Stokholm, J.; Bønnelykke, K.; Brix, S.; Bisgaard, H. Neonates with reduced neonatal lung function have systemic low-grade inflammation. J. Allergy Clin. Immunol. 2015, 135, 1450–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thysen, A.H.; Waage, J.; Larsen, J.M.; Rasmussen, M.A.; Stokholm, J.; Chawes, B.; Fink, N.R.; Pedersen, T.M.; Wolsk, H.; Thorsteinsdottir, S.; et al. Distinct immune phenotypes in infants developing asthma during childhood. Sci. Transl. Med. 2020, 12, eaaw0258. [Google Scholar] [CrossRef] [PubMed]
- Yassour, M.; Vatanen, T.; Siljander, H.; Hämäläinen, A.-M.; Härkönen, T.; Ryhänen, S.J.; Franzosa, E.A.; Vlamakis, H.; Huttenhower, C.; Gevers, D.; et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci. Transl. Med. 2016, 8, 343ra81. [Google Scholar] [CrossRef] [Green Version]
- Durack, J.; Christophersen, C.T. Human Respiratory and Gut Microbiomes—Do They Really Contribute to Respiratory Health? Front. Pediatr. 2020, 8, 528. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.-C.; Stiemsma, L.T.; Dimitriu, P.A.; Thorson, L.; Russell, S.; Yurist-Doutsch, S.; Kuzeljevic, B.; Gold, M.J.; Britton, H.M.; Lefebvre, D.L.; et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci. Transl. Med. 2015, 7, 307ra152. [Google Scholar] [CrossRef] [PubMed]
- Stokholm, J.; Blaser, M.J.; Thorsen, J.; Rasmussen, M.A.; Waage, J.; Vinding, R.K.; Schoos, A.-M.M.; Kunøe, A.; Fink, N.R.; Chawes, B.L.; et al. Maturation of the gut microbiome and risk of asthma in childhood. Nat. Commun. 2018, 9, 141. [Google Scholar] [CrossRef] [PubMed]
- Olin, A.; Henckel, E.; Chen, Y.; Lakshmikanth, T.; Pou, C.; Mikes, J.; Gustafsson, A.; Bernhardsson, A.K.; Zhang, C.; Bohlin, K.; et al. Stereotypic Immune System Development in Newborn Children. Cell 2018, 174, 1277–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depner, M.; PASTURE Study Group; Taft, D.H.; Kirjavainen, P.V.; Kalanetra, K.M.; Karvonen, A.M.; Peschel, S.; Schmausser-Hechfellner, E.; Roduit, C.; Frei, R.; et al. Maturation of the gut microbiome during the first year of life contributes to the protective farm effect on childhood asthma. Nat. Med. 2020, 26, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- LeVan, S.R.; Stamnes, K.A.; Lin, D.L.; Panzer, A.R.; Fukui, E.; McCauley, K.; Fujimura, K.E.; Mckean, M.; Ownby, D.R.; Zoratti, E.M.; et al. Elevated faecal 12,13-diHOME concentration in neonates at high risk for asthma is produced by gut bacteria and impedes immune tolerance. Nat. Microbiol. 2019, 4, 1851–1861. [Google Scholar] [CrossRef]
- Antunes, K.H.; Fachi, J.L.; De Paula, R.; Da Silva, E.F.; Pral, L.P.; Dos Santos, A. Áurea; Dias, G.B.M.; Vargas, J.E.; Puga, R.; Mayer, F.Q.; et al. Microbiota-derived acetate protects against respiratory syncytial virus infection through a GPR43-type 1 interferon response. Nat. Commun. 2019, 10, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Diaz, A.; Garcia-Maurino, C.; Jordan-Villegas, A.; Naples, J.; Ramilo, O.; Mejias, A. Viral Bacterial Interactions in Children: Impact on Clinical Outcomes. Pediatr. Infect. Dis. J. 2019, 38, S14–S19. [Google Scholar] [CrossRef]
- Bisgaard, H.; Hermansen, M.N.; Bønnelykke, K.; Stokholm, J.; Baty, F.; Skytt, N.L.; Aniscenko, J.; Kebadze, T.; Johnston, S.L. Association of bacteria and viruses with wheezy episodes in young children: Prospective birth cohort study. BMJ 2010, 341, c4978. [Google Scholar] [CrossRef] [Green Version]
- Mansbach, J.M.; Luna, P.N.; Shaw, C.A.; Hasegawa, K.; Petrosino, J.F.; Piedra, P.A.; Sullivan, A.F.; Espinola, J.A.; Stewart, C.J.; Camargo, C.A. Increased Moraxella and Streptococcus species abundance after severe bronchiolitis is associated with recurrent wheezing. J. Allergy Clin. Immunol. 2020, 145, 518–527.e8. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, X.; Zhang, N.; Wang, X.; Sun, L.; Chen, N.; Zhao, S.; He, Q. Airway microbiome, host immune response and recurrent wheezing in infants with severe respiratory syncytial virus bronchiolitis. Pediatr. Allergy Immunol. 2019, 31, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Salazar, C.; Shilts, M.H.; Tovchigrechko, A.; Schobel, S.; Chappell, J.D.; Larkin, E.K.; Gebretsadik, T.; Halpin, R.A.; Nelson, K.E.; Moore, M.L.; et al. Nasopharyngeal Lactobacillus is associated with a reduced risk of childhood wheezing illnesses following acute respiratory syncytial virus infection in infancy. J. Allergy Clin. Immunol. 2018, 142, 1447–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piters, W.D.S.; Heinonen, S.; Hasrat, R.; Bunsow, E.; Smith, B.; Suarez-Arrabal, M.-C.; Chaussabel, D.; Cohen, D.M.; Sanders, E.A.M.; Ramilo, O.; et al. Nasopharyngeal Microbiota, Host Transcriptome, and Disease Severity in Children with Respiratory Syncytial Virus Infection. Am. J. Respir. Crit. Care Med. 2016, 194, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Habibi, M.S.; Thwaites, R.S.; Chang, M.; Jozwik, A.; Paras, A.; Kirsebom, F.; Varese, A.; Owen, A.; Cuthbertson, L.; James, P.; et al. Neutrophilic inflammation in the respiratory mucosa predisposes to RSV infection. Science 2020, 370, eaba9301. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Z.; Wraith, A.; Bowden, J.; Alpers, J.; Forsyth, K.; Xu, H. Neutrophils induce damage to respiratory epithelial cells infected with respiratory syncytial virus. Eur. Respir. J. 1998, 12, 612–618. [Google Scholar] [CrossRef] [Green Version]
- Tahamtan, A.; Besteman, S.; Samadizadeh, S.; Rastegar, M.; Bont, L.; Salimi, V. Neutrophils in respiratory syncytial virus infection: From harmful effects to therapeutic opportunities. Br. J. Pharmacol. 2021, 178, 515–530. [Google Scholar] [CrossRef]
- Man, W.H.; Scheltema, N.M.; Clerc, M.; van Houten, M.A.; Nibbelke, E.; Achten, N.B.; Arp, K.; Sanders, E.A.M.; Bont, L.J.; Bogaert, D. Infant respiratory syncytial virus prophylaxis and nasopharyngeal microbiota until 6 years of life: A subanalysis of the MAKI randomised controlled trial. Lancet Respir. Med. 2020, 8, 1022–1031. [Google Scholar] [CrossRef]
- Beigelman, A.; Isaacson-Schmid, M.; Sajol, G.; Baty, J.; Rodriguez, O.M.; Leege, E.; Lyons, K.; Schweiger, T.L.; Zheng, J.; Schechtman, K.B.; et al. Randomized trial to evaluate azithromycin’s effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J. Allergy Clin. Immunol. 2015, 135, 1171–1178. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Bacharier, L.B.; Isaacson-Schmid, M.; Baty, J.; Schechtman, K.B.; Sajol, G.; Wylie, K.; Storch, G.A.; Castro, M.; Beigelman, A. Azithromycin therapy during respiratory syncytial virus bronchiolitis: Upper airway microbiome alterations and subsequent recurrent wheeze. J. Allergy Clin. Immunol. 2016, 138, 1215–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stokholm, J.; Chawes, B.L.; Vissing, N.H.; Bjarnadóttir, E.; Pedersen, T.M.; Vinding, R.K.; Schoos, A.-M.M.; Wolsk, H.M.; Thorsteinsdóttir, S.; Hallas, H.W.; et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1–3 years: A randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2016, 4, 19–26. [Google Scholar] [CrossRef]
- Luisi, F.L.; Roza, C.A.; Silveira, V.D.; Machado, C.C.; Da Rosa, K.M.; Pitrez, P.M.; Jones, M.H.; Stein, R.T.; Leitão, L.A.D.A.; Comaru, T.; et al. Azithromycin administered for acute bronchiolitis may have a protective effect on subsequent wheezing. J. Bras. Pneumol. 2020, 46, e20180376. [Google Scholar] [CrossRef] [PubMed]
- Bacharier, L.B. Azithromycin During Wheezing Illnesses Among Preschool Children—Does the Airway Microbiota Provide Insights into Mechanism? Am. J. Respir. Crit. Care Med. 2021, 8. [Google Scholar] [CrossRef]
- Thorsen, J.; Stokholm, J.; Rasmussen, M.A.; Mortensen, M.S.; Brejnrod, A.D.; Hjelmsø, M.; Shah, S.; Chawes, B.; Bønnelykke, K.; Sørensen, S.J.; et al. The Airway Microbiota Modulates Effect of Azithromycin Treatment for Episodes of Recurrent Asthma-like Symptoms in Preschool Children: A Randomized Clinical Trial. Am. J. Respir. Crit. Care Med. 2021, 202008-3226. [Google Scholar] [CrossRef] [PubMed]
- Nieto, A.; Mazón, A.; Nieto, M.; Calderón, R.; Calaforra, S.; Selva, B.; Uixera, S.; Palao, M.J.; Brandi, P.; Conejero, L.; et al. Bacterial Mucosal Immunotherapy with MV130 Prevents Recurrent Wheezing in Children: A Randomized, Double-blind, Placebo-controlled Trial. Am. J. Respir. Crit. Care Med. 2021. [Google Scholar] [CrossRef]
- Hjelmsø, M.H.; Shah, S.A.; Thorsen, J.; Rasmussen, M.; Vestergaard, G.; Mortensen, M.S.; Brejnrod, A.; Brix, S.; Chawes, B.; Bønnelykke, K.; et al. Prenatal dietary supplements influence the infant airway microbiota in a randomized factorial clinical trial. Nat. Commun. 2020, 11, 426-10. [Google Scholar] [CrossRef]
- Stokholm, J.; Thorsen, J.; Blaser, M.J.; Rasmussen, M.A.; Hjelmsø, M.; Shah, S.; Christensen, E.D.; Chawes, B.L.; Bønnelykke, K.; Brix, S.; et al. Delivery mode and gut microbial changes correlate with an increased risk of childhood asthma. Sci. Transl. Med. 2020, 12, eaax9929. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, M.S.; Rasmussen, M.A.; Stokholm, J.; Brejnrod, A.D.; Balle, C.; Thorsen, J.; Krogfelt, K.A.; Bisgaard, H.; Sørensen, S.J. Modeling transfer of vaginal microbiota from mother to infant in early life. eLife 2021, 10, e57051. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nino, G.; Rodriguez-Martinez, C.E.; Gutierrez, M.J. Early Microbial–Immune Interactions and Innate Immune Training of the Respiratory System during Health and Disease. Children 2021, 8, 413. https://0-doi-org.brum.beds.ac.uk/10.3390/children8050413
Nino G, Rodriguez-Martinez CE, Gutierrez MJ. Early Microbial–Immune Interactions and Innate Immune Training of the Respiratory System during Health and Disease. Children. 2021; 8(5):413. https://0-doi-org.brum.beds.ac.uk/10.3390/children8050413
Chicago/Turabian StyleNino, Gustavo, Carlos E. Rodriguez-Martinez, and Maria J. Gutierrez. 2021. "Early Microbial–Immune Interactions and Innate Immune Training of the Respiratory System during Health and Disease" Children 8, no. 5: 413. https://0-doi-org.brum.beds.ac.uk/10.3390/children8050413