Towards AI-Enabled Multimodal Diagnostics and Management of COVID-19 and Comorbidities in Resource-Limited Settings
Abstract
:1. Introduction
2. The Technological Challenges and Objectives of the Framework
2.1. The Technological Challenge
2.2. Objectives of the Proposed Framework
- Collection of real-world evidence, particularly on diagnosis and treatment of COVID-19 patients
- Creation of an integrated platform for harnessing data from various POC diagnostic methods, including medical imaging-based diagnostics and healthcare IT systems
- Effective decision-making based on screening algorithms informing differential diagnosis and management of COVID-19-associated comorbidities in community-based health services in RLS
- Mobile phone-based contact tracing for COVID 19 and associated comorbidities
- PSGT combining diagnostic RNA-based SARS-CoV-2 testing with DNA-based chronic disease screening for managing potential biochemical abnormalities caused by gene-environment interaction.
3. Materials and Methods
3.1. Requirements of the AI-Enabled Framework for Multimodal Diagnostics of COVID-19
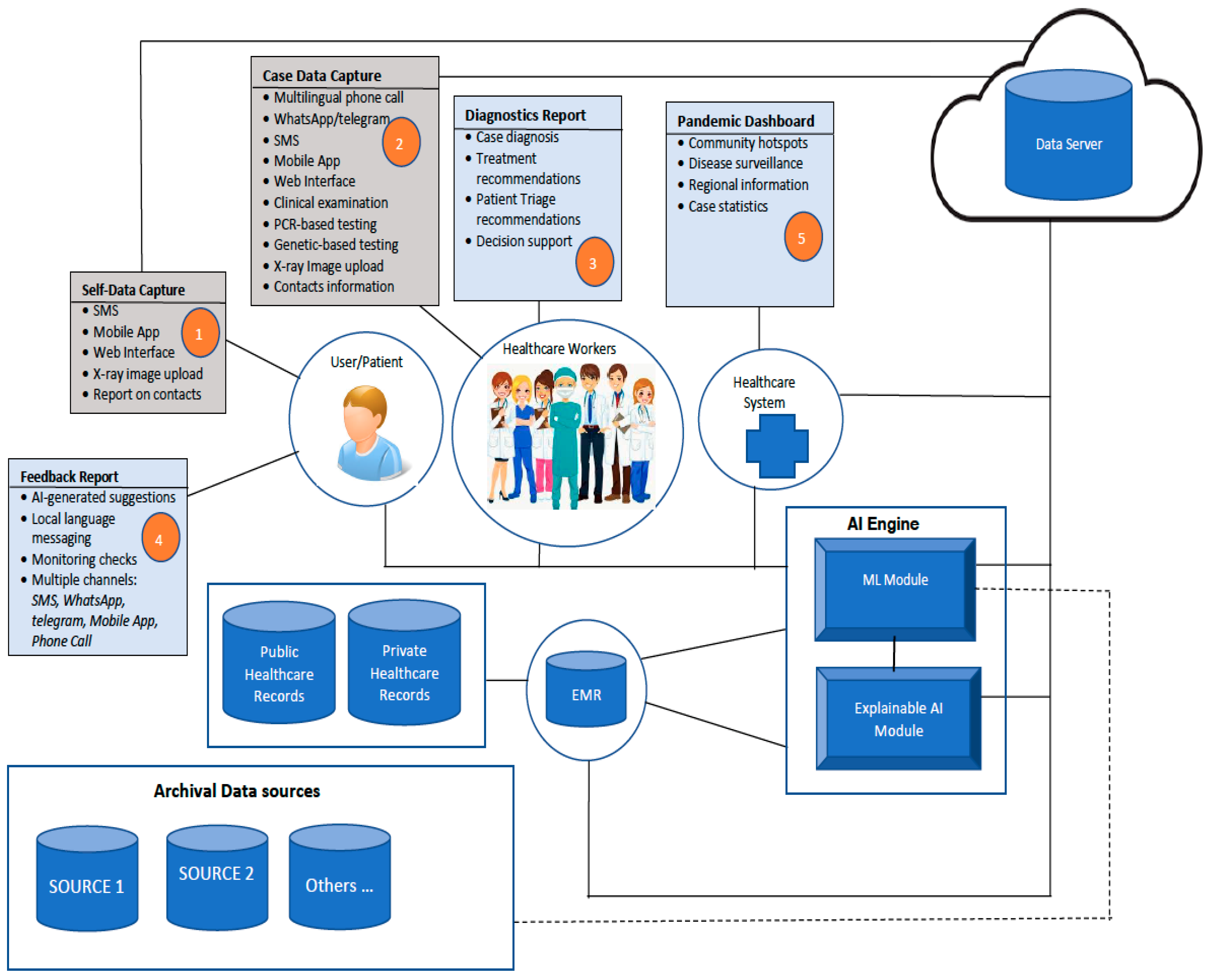
- Data collection from patients on symptoms of COVID-19 that have been observed. This will be performed by using diverse sources such as interaction with health care workers via the phone, SMS messaging, WhatsApp messages/call, Telegram message/call. The information collected will be limited to issues relating to COVID-19 and will be stored on a secure system. The security of electronic health records (EHR) during transmission from one point to another shall be secured by using credible encryption methods. The options to consider include the use of scrambled alpha-numeric randomization combined with RSA, as we did in a previous study [23].
- Case documentation by health care workers (HCW) through a mobile app, webform
- Capture of radiological images from patients
- Access and integration with electronic medical records
- Prediction of disease using machine learning (ML)
- AI-generated recommendations with explanations for HCW
- Delivery of AI-generated suggestions to patients through mobile phone via personal calls, SMS messaging, WhatsApp, Telegram, Mobile App. Messages can be conveyed directly through these means, or via a phone call by the HCW
- Regular monitoring of patients (under self-quarantine)
- Aiding decision-making on patient triage and treatment schedule
- Data analytics and reporting for pandemic management by health management systems (Dashboard reporting)—hotspots, cases per community, neighbourhoods, regions
- Contact tracing application based on Bluetooth technology that can track close contact with infected persons (only phone numbers are stored on patient’s mobile phone). The application will be triggered by user consent, and stored content is also shared by user consent.
3.2. Architecture of the Proposed Framework for Multimodal Diagnostics of COVID-19
3.3. Description of the Workflow and Configuration of the AI Engine
- a.
- Socio-demographic and lifestyle characteristics (e.g., age, gender, ethnicity, residential area, occupation, workplace, smoking status, alcohol use, drug use)
- b.
- Exposure history and symptoms (record of the previous contact with infected persons or places; symptoms types, level of severity of symptoms)
- c.
- Comorbidity profile (such as hypertension, COPD, diabetes, heart diseases, TB, and HIV, as well as severity level of the comorbidity)
- d.
- Medical imaging data such as CXR, chest CT of Mrs. AA
- e.
- Laboratory test data such as FBC, ferritin, PCT, MicroRNAs, NT-proBNP, and inflammatory cytokines)
- f.
- Data obtained for PSGT to customise feedback to Mrs. AA based on personal utility and clinical relevance (see Table 1).
- g.
- Vaccine and other Medication data (e.g., steroids, antibiotics, antiviral, antifungal, medication for glycaemic control, antihypertensive)
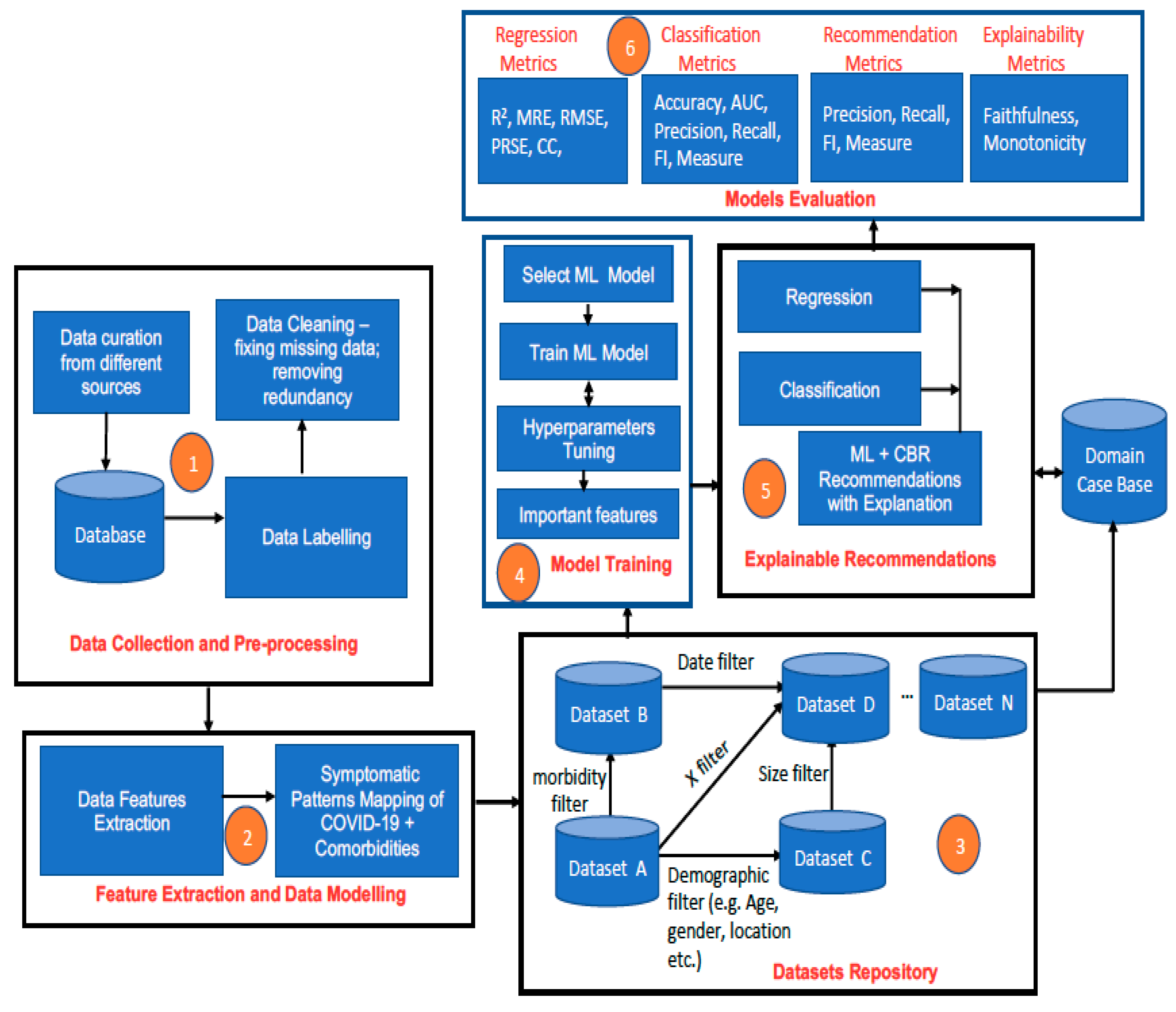
3.3.1. Data Collection and Pre-Processing
- i.
- Archived but anonymized data of patients (like Mrs. AA) with COVID-19 cases are collected from diverse sources—hospital records, medical records databases in South Africa. This similarly applies to any other country in SSA.
- ii.
- The collected data are labelled by using descriptive attribute names that depict the information that they represent. For example, if the objective is to predict the possibility of COVID-19 infection, COVID-19 death, or COVID-19 recovery, then the dependent attributes such as age profile, gender type, symptom severity profile, comorbidity profile, the severity of comorbidity disease, weight profile, blood profile, genetic data, etc. that can influence specific patient outcomes such as infection, death, recovery, survival must be identified and appropriately labelled. The process will be a combination of manual and automated data labelling depending on the inherent quality of collected initial data.
- iii.
- Data pre-processing will be performed to clean up the data, fix missing values, and balance the dataset (if necessary) to reduce bias. Instances of missing numeric values can be replaced by the median or the mean value (for data with a normal distribution), while missing categorical values can be replaced by the mode. When there is an imbalanced dataset due to the under-representation of a specific class, class balancing techniques such as the synthetic minority oversampling technique (SMOTE) [24] will be used to generate additional synthetic data to ensure a balanced dataset.
3.3.2. Feature Extraction and Data Modelling
- iv.
- Data points of interest (features) are extracted from the main database. This will include important biodata such as age, gender, address, demographic data, and key clinical information that pertain to the patient category concerned.
- v.
- Mapping of observed symptoms in patients with COVID-19 and specific morbidities will be performed. This will entail documenting symptoms that are associated with particular instances of COVID-19 and comorbidity. This will seek to answer the question of how the symptomatic patterns of COVID-19 plus HIV are different/similar to those of COVID-19 plus diabetes, for example. This process will allow different patients’ symptomatic profiles to be generated, e.g.:
- COVID-19 + HIV; COVID-19 + TB symptoms
- COVID-19 + HIV +TB symptoms; COVID-19 + diabetes (DB) symptoms
- COVID-19 + hypertension symptoms; COVID-19 + hypertension + DB
- COVID-19 + malaria; COVID-19 + malaria + HIV; COVID-19 + etc.
3.3.3. Dataset Repository
- vi.
- Based on the feature extraction in Step 4 and Step 5, different datasets will be created. It will be possible to filter the created datasets based on some parameters such as date, type of morbidity, age, demography, symptomatic pattern, and other dimensions that are of interest in order to create sub-datasets and variant datasets by using querying techniques. Data can be extracted using SQL statements to create sub-datasets. Examples of different SQL queries that can be used to extract different sub-datasets are shown in Table 2.
3.3.4. Model Training
- vii.
- Supervised machine learning algorithms (ANN, SVM, deep neural networks, ensemble models—random forest, extreme gradient boosted trees (XGBoost), bagging-gradient boosted trees (B-GBT), etc.) are trained based on selected datasets from the dataset repository and the type of prediction that is required to be made per time. Appropriate hyperparameter tuning methods such as cross-validation and regularisation shall be applied to prevent overfitting during training and better generalization of trained ML models. Methods such as k-fold validation, dropout regularization, ridge regression regularization (L2-norm), lasso regression regularization (L1-norm), entropy regularization, and noise injection are good options that shall be explored to achieve this [25]. Experiments on batch training using gradient descent algorithms and minibatch training with stochastic gradient descent using different activation functions shall also be performed to ensure that the most efficient methods are used for training so that the best-trained models are eventually used for predictions [26].
- viii.
- Trained predictive models are stored in the cloud-based machine learning server.
- ix.
- The most important features of trained models (highest weighted) are also stored. For tree-based ML models such as decision tree, random forest, extreme gradient boosted trees (XGBoost), and bagging-gradient boosted trees (B-GBT), this can be obtained from the feature importance property of the tree model [27]. For regression models, this could be obtained by using univariate selection, while for other ML models, dimensionality reduction methods such as principal component analysis (PCA) can be used to determine feature selection and feature importance [28]. Knowing the most important features of an ML model is particularly useful for the explainability of the ML model, as it reveals the features that have the strongest relationships with the output variable the most [29].
3.3.5. Real-Time Explainable ML-CBR Recommendations by the AI Engine
- x.
- New case data is received that requires prediction.
- xi.
- Required AI operation is selected by the user (HCW). This could be a regression task to estimate something, classify/identify something, or obtain a recommendation.
- xii.
- ML prediction is generated by the AI engine.
- xiii.
- The result of the ML algorithm is sent to a case-based reasoning module (CBR).
- CBR uses the most important features as a basis to retrieve similar cases to the current case from the case base. The case base is linked to the original dataset used to train the ML model.
- The feature values of cases that are similar to the current case are used to construct an explanation to justify the prediction generated by the ML model, thereby utilizing a post hoc approach to ensure the explainability of the ML result.
- xiv.
- Every predicted case is stored in the case base for future reuse.
3.3.6. Model Evaluation
3.4. Utilization of Generated Results
4. The Merits of the Proposed Framework
- Harness the different types of clinically relevant data for decision-making on diagnosis and treatment of COVID-19 and comorbidities;
- Enable differentiated and personalised treatment for patients with COVID-19 and comorbidities in terms of patient triage, treatment, and post-infection management;
- Use multimodal diagnostic tools to guide the management of patients with COVID-19 and comorbidities (HIV/TB/malaria) during treatment, determining the pattern of disease progression and handling of posttreatment complications;
- Use clinical diagnosis from different disciplines to guide management of PRDs in the presence of COVID-19;
- Determine the possibility of patients with comorbidity developing COVID-19 if there is exposure due to their risk factors;
- Generate intelligent and explanation-rich recommendations on detected cases for use by healthcare workers (HCW) to aid decision-making, thus enabling trustworthy AI for healthcare;
- Support the process of disease surveillance in RLS through multi-sensory contact tracing, thus helping with outbreak control;
- Generate timely analytical reports on key factors that influence spread, infection recovery rates, and other factors of interest; and
- Aid the formulation of policies and strategies for containing and combating COVID-19.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davies, M.-A. HIV and risk of COVID-19 death: A population cohort study from the Western Cape Province, South Africa. medRxiv 2020. [Google Scholar] [CrossRef]
- COVID Live Update: 166,632,552 Cases and 3,460,798 Deaths from the Coronavirus—Worldometer. Available online: https://www.worldometers.info/coronavirus/ (accessed on 22 May 2021).
- TB Facts—Tests, Drugs, Statistics & Lots more about TB Disease. Available online: http://www.tbfacts.org (accessed on 22 May 2021).
- TB in Nigeria—Funding, Children, Diagnosing TB, HIV/TB. Available online: https://tbfacts.org/tb-nigeria/ (accessed on 22 May 2021).
- Chehade, M.J.; Yadav, L.; Jayatilaka, A.; Gill, T.K.; Palmer, E. Personal digital health hubs for multiple conditions. Bull. World Health Organ. 2020, 98, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Moyo, S.; Boffa, J.; Mhlaba, T.; Sulis, G.; Sifumba, Z.; Pai, M.; Daftary, A. COVID-19 and tuberculosis in South Africa: A dangerous combination. S. Afr. Med. J. 2020, 110, 341–342. [Google Scholar]
- Kavanagh, M.M.; Erondu, N.A.; Tomori, O.; Dzau, V.J.; Okiro, E.A.; Maleche, A.; Aniebo, I.C.; Rugege, U.; Holmes, C.B.; Gostin, L.O. Access to lifesaving medical resources for African countries: COVID-19 testing and response, ethics, and politics. Lancet 2020, 395, 1735–1738. [Google Scholar] [CrossRef]
- Mashamba-Thompson, T.P.; Crayton, E.D. Blockchain and artificial intelligence technology for novel Coronavirus disease-19 self-testing. Diagnostics 2020, 10, 198. [Google Scholar] [CrossRef] [Green Version]
- Daramola, O.; Moser, T. Semantic integration of multiple health data for treatment decision-making in low-resource settings. In Proceedings of the Multi Conference on Computer Science and Information Systems. In Proceedings of the MCCSIS 2019—Proceedings of the International Conference on e-Health 2019, Porto, Portugal, 16–19 July 2019. [Google Scholar]
- Stroetmann, K.A. Digital Health Ecosystems for African Countries—A Guide for Public and Private Actors for Establishing Holistic Digital Health Ecosystems in Africa; Federal Ministry for Economic Cooperation and Development and Strategic: Bonn, Germany, 2018. [Google Scholar]
- Kotze, M.J.; Van Velden, D.P.; Botha, K.; Badenhorst, C.H.; Avenant, H.; Van Rensburg, S.J.; Cronjé, F.J. Pathology-supported genetic testing directed at shared disease pathways for optimized health in later life. Per. Med. 2013, 10, 497–507. [Google Scholar] [CrossRef]
- Kwok, S.; Adam, S.; Ho, J.H.; Iqbal, Z.; Turkington, P.; Razvi, S.; Le Roux, C.W.; Soran, H.; Syed, A.A. Obesity: A critical risk factor in the COVID-19 pandemic. Clin. Obes. 2020, 10, e12403. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Geng, Z.; Fan, Z.; Liu, J.; Chen, H. Point-of-care testing based on smartphone: The current state-of-the-art (2017–2018). Biosens. Bioelectron. 2019, 132, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Dolan, M.E.; Hill, D.P.; Mukherjee, G.; McAndrews, M.S.; Chesler, E.J.; Blake, J.A. Investigation of COVID-19 comorbidities reveals genes and pathways coincident with the SARS-CoV-2 viral disease. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kotze, M. Application of advanced molecular technology in the diagnosis and management of genetic disorders in South Africa. S. Afr. Med. J. 2016, 106, S114–S118. [Google Scholar] [CrossRef] [Green Version]
- Cavanaugh, A.M.; Spicer, K.B.; Thoroughman, D.; Glick, C.; Winter, K. Reduced risk of reinfection with SARS-CoV-2 after COVID-19 vaccination—Kentucky, May–June 2021. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 1081–1083. [Google Scholar] [CrossRef] [PubMed]
- Lückhoff, H.K.; Kidd, M.; van Rensburg, S.J.; van Velden, D.P.; Kotze, M.J. Apolipoprotein E genotyping and questionnaire-based assessment of lifestyle risk factors in dyslipidemic patients with a family history of Alzheimer’s disease: Test development for clinical application. Metab. Brain Dis. 2016, 31, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Marais, A.D.; Kotze, M.J.; Raal, F.J.; Khine, A.A.; Talmud, P.J.; Humphries, S.E. Familial hypercholesterolaemia workshop for leveraging point-of-care testing and personalised medicine in association with the Lipid and Atherosclerosis Society of Southern Africa. Cardiovasc. J. Afr. 2019, 30, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Wyller, T.B.; Kittang, B.R.; Ranhoff, A.H.; Harg, P.; Myrstad, M. Nursing home deaths after COVID-19 vaccination. Tidsskr. Nor. Laegeforen. 2021, 141. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Karathanasis, S.K.; Yang, Z.-H.; Freeman, L.; Kotani, K.; Remaley, A.T. COVID-19-Associated dyslipidemia: Implications for mechanism of impaired resolution and novel therapeutic approaches. FASEB J. 2020, 34, 9843–9853. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, M.; Bedi, O.; Gupta, M.; Kumar, S.; Jaiswal, G.; Rahi, V.; Yedke, N.G.; Bijalwan, A.; Sharma, S.; et al. Role of vitamins and minerals as immunity boosters in COVID-19. Inflammopharmacology 2021, 29, 1001–1016. [Google Scholar] [CrossRef]
- Kuo, C.L.; Pilling, L.C.; Atkins, J.L.; Masoli, J.A.; Delgado, J.; Kuchel, G.A.; Melzer, D. APOE e4 genotype predicts severe COVID-19 in the UK biobank community cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 2231–2232. [Google Scholar] [CrossRef]
- Osamor, V.C.; Edosomwan, I.B. Employing scrambled alpha-numeric randomization and RSA algorithm to ensure enhanced encryption in electronic medical records. Inform. Med. Unlocked. 2021, 25, 100672. [Google Scholar] [CrossRef]
- Gonzalez-Cuautle, D.; Hernandez-Suarez, A.; Sanchez-Perez, G.; Toscano-Medina, L.K.; Portillo-Portillo, J.; Olivares-Mercado, J.; Perez-Meana, H.M.; Sandoval-Orozco, A.L. Synthetic Minority Oversampling Technique for optimizing classification tasks in botnet and intrusion-detection-system datasets. Appl. Sci. 2020, 10, 794. [Google Scholar] [CrossRef] [Green Version]
- Nusrat, I.; Jang, S.-B. A comparison of regularization techniques in deep neural networks. Symmetry 2018, 10, 648. [Google Scholar] [CrossRef] [Green Version]
- Domingos, E.; Ojeme, B.; Daramola, O. Experimental analysis of hyperparameters for deep learning-based churn prediction in the banking sector. Computation 2021, 9, 34. [Google Scholar] [CrossRef]
- Brownlee, J. Data Preparation for Machine Learning: Data Cleaning, Feature Selection, and Data Transforms in Python; Machine Learning Mastery: San Francisco, CA, USA, 2020. [Google Scholar]
- Khalid, S.; Khalil, T.; Nasreen, S. A survey of feature selection and feature extraction techniques in machine learning. In Proceedings of the 2014 Science and Information Conference, London, UK, 27–29 August 2014; IEEE: Manhattan, NY, USA, 2014. [Google Scholar]
- Saarela, M.; Jauhiainen, S. Comparison of feature importance measures as explanations for classification models. SN Appl. Sci. 2021, 3, 1–12. [Google Scholar] [CrossRef]
| Weight Management and Your Health | |
|---|---|
| Overweight-BMI > 26 kg/m2 | Your body mass index (BMI) that provides a measure of height in relation to weight (kg/m2) is above the ideal range of between 18.5 and 24.9 kg/m2. The BMI is only one indicator of a healthy weight and should therefore be monitored together with skinfold measurements and the percentage body fat to achieve and maintain your target weight. |
| Chronic inflammation | Fat accumulation needs to be avoided as it is accompanied by low-grade inflammation, which results in resistance to weight loss. This is very important due to the detection of a genetic variation (APOE e4 allele) involved in both cholesterol/fat metabolism and inflammation. |
| Metabolic syndrome risk | Being overweight or obese is associated with an increased risk for the metabolic syndrome, which is caused by a combination of 3 or more of the following features: central obesity, hypertension, insulin resistance and/or dyslipidaemia as may be reflected by high triglycerides and/or low HDL-cholesterol levels. |
| Optimise Your Diet | |
| Fat intake-Low (excellent) | Maintain the overall low intake of fat reported. High total fat, especially trans-fats and saturated fat intake, correlates with increased body weight and an abnormal lipid profile. Incorporate healthy fats in your diet regularly. Phospholipids will help maintain brain health, which is of concern due to the genetic variation detected. Phospholipid supplements in the form of lecithin or its other forms, phosphatidylcholine and phosphatidylserine, are available. Good food sources are milk, shellfish, and egg yolk. |
| Folate intake-Low | The daily amount of folate required in the diet differs between individuals according to their genetic make-up and health status. The low folate score (<14) identified correlates with increased homocysteine and body mass index. Ensure a minimum intake of 400 ug folate per day together with adequate amounts of vitamin B6, B12, and riboflavin. Green leafy vegetables (e.g., spinach, broccoli, asparagus), fresh orange juice, and dried beans are good sources. |
| Improve Your Lifestyle | |
| Physical activity-Moderate | Ensure regular participation in sporting activities (e.g., running, jogging, walking, swimming) and/or follow a moderate exercise program for at least 30–60 min most days of the week. Physical activity promotes blood circulation and vascular health aimed at prevention of dementia/Alzheimer’s disease with advanced aging, especially in genetically susceptible individuals. |
| Monitor Your Blood Levels | |
| Lipid Profile-Not provided | Keeping cholesterol and triglyceride levels within the normal ranges is very important due to a genetic hyper-responsiveness to lifestyle risk factors. Measurement of these levels is recommended to determine gene expression and to monitor response to treatment. |
| Optimise Your Treatment Program | |
| Medical History (Reported at referral in 2011) | Family Medical Conditions:
|
| Genetic variation affecting inflammation/blood clotting | Intake of at least 1–2 g omega-3 fatty acids per day may be advisable, especially when intake of dietary sources (e.g., oily fish) is inadequate. A ratio of 2:1 to 3:1 for omega-6 and 3 is recommended. Intake of omega-6 is generally too high in most populations; therefore, fish or fish oil supplements are the preferred sources for increasing omega-3 fatty acid intake when omega-6 intake is adequate. Certain foods such as milk and eggs are fortified with omega-3s and are also recommended. It is important to note that supplementation with omega-3 fatty acids and other nutrients may affect the response to certain medications (e.g., warfarin, chemotherapy). |
| Genetic variation indicating altered nutritional requirements for your genotype | Adequate daily intake of vitamins and minerals [21] required as enzyme co-factors with additional intake of antioxidants is advisable in the presence of the APOE gene variation linked to COVID-19 infection, severity, and co-morbidities [22]. |
| S/n | Task | Query Statements |
|---|---|---|
| 1 | Extract data on cases of COVID-19 + TB | SELECT All from Database where Case = ‘COVID-19′ AND comorbidity = ‘TB’ |
| 2 | Extract data on cases of COVID-19 + HIV + TB | SELECT All from Database where Case = ‘COVID-19′ AND (comorbidity = ‘TB’ AND comorbidity = ‘HIV’) |
| 3 | Extract data on cases of COVID-19 + comorbidity for males that are 60 years and older | SELECT All from Database where Case = ‘COVID-19′ AND (comorbidity = ‘TB’ OR comorbidity = ‘HIV’ OR comorbidity = ‘Diabetes’ OR comorbidity = ‘Hypertension’ OR comorbidity = ‘Malaria’) AND (gender = ‘Male’) AND (age ≥ 60) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daramola, O.; Nyasulu, P.; Mashamba-Thompson, T.; Moser, T.; Broomhead, S.; Hamid, A.; Naidoo, J.; Whati, L.; Kotze, M.J.; Stroetmann, K.; et al. Towards AI-Enabled Multimodal Diagnostics and Management of COVID-19 and Comorbidities in Resource-Limited Settings. Informatics 2021, 8, 63. https://0-doi-org.brum.beds.ac.uk/10.3390/informatics8040063
Daramola O, Nyasulu P, Mashamba-Thompson T, Moser T, Broomhead S, Hamid A, Naidoo J, Whati L, Kotze MJ, Stroetmann K, et al. Towards AI-Enabled Multimodal Diagnostics and Management of COVID-19 and Comorbidities in Resource-Limited Settings. Informatics. 2021; 8(4):63. https://0-doi-org.brum.beds.ac.uk/10.3390/informatics8040063
Chicago/Turabian StyleDaramola, Olawande, Peter Nyasulu, Tivani Mashamba-Thompson, Thomas Moser, Sean Broomhead, Ameera Hamid, Jaishree Naidoo, Lindiwe Whati, Maritha J. Kotze, Karl Stroetmann, and et al. 2021. "Towards AI-Enabled Multimodal Diagnostics and Management of COVID-19 and Comorbidities in Resource-Limited Settings" Informatics 8, no. 4: 63. https://0-doi-org.brum.beds.ac.uk/10.3390/informatics8040063







