Processing of Carob Kernels to Syrup by Ultrasound-Assisted Extraction
Abstract
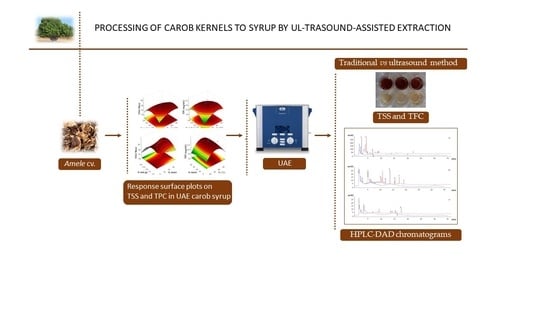
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals
2.3. Carob Syrup Obtained by a Traditional Method
2.4. Carob Syrup Obtained by the Ultrasound-Assisted Extraction Process
2.5. TSS and TPC Determination
2.6. HPLC-DAD Analysis
2.7. Experimental Design and Statistical Analyses
3. Results and Discussion
3.1. Optimization of UAE Parameters by CCD-RSM for Alternative Carob Syrup Preparation
3.2. HPLC-DAD Analysis of Polyphenols in Carob Syrup
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stavrou, I.J.; Christou, A.; Kapnissi-Christodoulou, C.P. Polyphenols in carobs: A review on their composition, antioxidant capacity and cytotoxic effects, and health impact. Food Chem. 2018, 269, 355–374. [Google Scholar] [CrossRef] [PubMed]
- van Rijs, P.; Fogliano, V. Roasting carob flour decreases the capacity to bind glycoconjugates of bile acids. Food Funct. 2020, 11, 5924–5932. [Google Scholar] [CrossRef] [PubMed]
- Ayache, S.B.; Reis, F.S.; Dias, M.I.; Pereira, C.; Glamočlija, J.; Soković, M.; Behija Saafi, E.; Ferreira, I.C.F.R.; Barros, L.; Achour, L. Chemical characterization of carob seeds (Ceratonia siliqua L.) and use of different extraction techniques to promote its bioactivity. Food Chem. 2021, 351, 129263. [Google Scholar] [CrossRef] [PubMed]
- Goulas, V.; Stylos, E.; Chatziathanasiadou, M.V.; Mavromoustakos, T.; Tzakos, A.G. Functional components of carob fruit: Linking the chemical and biological space. Int. J. Mol. Sci. 2016, 17, 1875. [Google Scholar] [CrossRef] [PubMed]
- Barak, S.; Mudgil, D. Locust bean gum: Processing, properties and food applications—A review. Int. J. Biol. Macromol. 2014, 66, 74–80. [Google Scholar] [CrossRef]
- Dakia, P.A.; Wathelet, B.; Paquot, M. Isolation and chemical evaluation of carob (Ceratonia siliqua L.) seed germ. Food Chem. 2007, 102, 1368–1374. [Google Scholar] [CrossRef]
- Farha, A.K.; Yang, Q.Q.; Kim, G.; Li, H.B.; Zhu, F.; Liu, H.Y.; Gan, R.Y.; Corke, H. Tannins as an alternative to antibiotics. Food Biosci. 2020, 38, 100751. [Google Scholar] [CrossRef]
- Sultana, S.; Khan, A.; Safhi, M.M.; Alhazmi, H.A. Cough suppressant herbal drugs: A review. Int. J. Pharm. Sci. Invent. 2016, 5, 15–28. [Google Scholar]
- Dhaouadi, K.; Belkhir, M.; Akinocho, I.; Raboudi, F.; Pamies, D.; Barrajón, E.; Estevan, C.; Fattouch, S. Sucrose supplementation during traditional carob syrup processing affected its chemical characteristics and biological activities. LWT-Food Sci. Technol. 2014, 57, 1–8. [Google Scholar] [CrossRef]
- Papaefstathiou, E.; Agapiou, A.; Giannopoulos, S.; Kokkinofta, R. Nutritional characterization of carobs and traditional carob products. Food Sci. Nutr. 2018, 6, 2151–2161. [Google Scholar] [CrossRef]
- Roseiro, L.B.; Duarte, L.C.; Oliveira, D.L.; Roque, R.; Bernardo-Gil, M.G.; Martins, A.I.; Sepúlveda, C.; Almeida, J.; Meireles, M.; Gírio, F.M.; et al. Supercritical, ultrasound and conventional extracts from carob (Ceratonia siliqua L.) biomass: Effect on the phenolic profile and antiproliferative activity. Ind. Crops Prod. 2013, 47, 132–138. [Google Scholar] [CrossRef]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Crupi, P.; Dipalmo, T.; Clodoveo, M.L.; Toci, A.T.; Coletta, A. Seedless table grape residues as a source of polyphenols: Comparison and optimization of non-conventional extraction techniques. Eur. Food Res. Technol. 2018, 244, 1091–1100. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Crupi, P.; Muraglia, M.; Corbo, F. Ultrasound Assisted Extraction of Polyphenols from Ripe Carob Pods (Ceratonia siliqua L.): Combined Designs for Screening and Optimizing the Processing Parameters. Foods 2022, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Ranjha, M.M.A.; Irfan, S.; Lorenzo, J.M.; Shafique, B.; Kanwal, R.; Pateiro, M.; Arshad, R.N.; Wang, L.; Nayik, G.A.; Roobab, U.; et al. Sonication, a potential technique for extraction of phytoconstituents: A systematic review. Processes 2021, 9, 1406. [Google Scholar] [CrossRef]
- Zia, S.; Khan, M.R.; Shabbir, M.A.; Aslam Maan, A.; Khan, M.K.I.; Nadeem, M.; Khalild, A.A.; Din, A.; Aadil, R.M. An inclusive overview of advanced thermal and nonthermal extraction techniques for bioactive compounds in food and food-related matrices. Food Rev. Int. 2020, 36, 1–31. [Google Scholar] [CrossRef]
- El Batal, H.; Hasib, A.; Bacaoui, A.; Dehbi, F.; Ouatmane, A.; Jaouad, A. Syrup of natural carob sugars and a process for its production using Response Surface Methodology. Chem. Process Eng. Res. 2013, 10, 44–50. [Google Scholar]
- Milani, G.; Curci, F.; Cavalluzzi, M.M.; Crupi, P.; Pisano, I.; Lentini, G.; Clodoveo, M.L.; Franchini, C.; Corbo, F. Optimization of Microwave-Assisted Extraction of Antioxidants from Bamboo Shoots of Phyllostachys pubescens. Molecules 2020, 25, 215. [Google Scholar] [CrossRef] [Green Version]
- El Batal, H.; Hasib, A.; Ouatmane, A.; Dehbi, F.; Jaouad, A.; Boulli, A. Sugar composition and yield of syrup production from the pulp of Moroccan carob pods (Ceratonia siliqua L.). Arab. J. Chem. 2016, 9, S955–S959. [Google Scholar] [CrossRef] [Green Version]
- Petit, M.D.; Pinilla, J.M. Production and purification of a sugar syrup from carob pods. LWT-Food Sci. Technol. 1995, 28, 145–152. [Google Scholar] [CrossRef]
- Clark, C.; Williges, R.C. Response surface methodology central-composite design modifications for human performance research. Human Factors 1973, 15, 295–310. [Google Scholar] [CrossRef]
- Christou, A.; Stavrou, I.J.; Kapnissi-Christodoulou, C.P. Continuous and pulsed ultrasound-assisted extraction of carob’s antioxidants: Processing parameters optimization and identification of polyphenolic composition. Ultrason. Sonochem. 2021, 76, 105630. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. TrAC Trends Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Katsube, T.; Tsurunaga, Y.; Sugiyama, M.; Furuno, T.; Yamasaki, Y. Effect of air-drying temperature on antioxidant capacity and stability of polyphenolic compounds in mulberry (Morus alba L.) leaves. Food Chem. 2009, 113, 964–969. [Google Scholar] [CrossRef]
- De Taeye, C.; Kankolongo Cibaka, M.L.; Jerkovic, V.; Collin, S. Degradation of (−)-epicatechin and procyanidin B2 in aqueous and lipidic model systems. First evidence of “chemical” flavan-3-ol oligomers in processed cocoa. J. Agric. Food Chem. 2014, 62, 9002–9016. [Google Scholar] [CrossRef] [PubMed]
- Lončarić, A.; Pablo Lamas, J.; Guerra, E.; Kopjar, M.; Lores, M. Thermal stability of catechin and epicatechin upon disaccharides addition. Int. J. Food Sci. Technol. 2018, 53, 1195–1202. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, J.; Xu, D.; Wang, S.; Yuan, Y.; Cao, Y. Investigation of (+)−catechin stability under ultrasonic treatment and its degradation kinetic modeling. J. Food Process Eng. 2018, 41, e12904. [Google Scholar] [CrossRef]
- Qiao, L.; Ye, X.; Sun, Y.; Ying, J.; Shen, Y.; Chen, J. Sonochemical effects on free phenolic acids under ultrasound treatment in a model system. Ultrason. Sonochem. 2013, 20, 1017–1025. [Google Scholar] [CrossRef]





| Experiments | Block | X1 (°C) | X2 (min) | X3 (mL/g) | TSS (°Brix) | TPC (mg/mL) |
|---|---|---|---|---|---|---|
| 8 | 1 | 45 | 17 | 2.5 | 18.0 | 0.998 |
| 3 | 1 | 25 | 17 | 1.5 | 20.0 | 1.042 |
| 1 | 1 | 25 | 8 | 1.5 | 22.2 | 0.869 |
| 4 | 1 | 25 | 17 | 2.5 | 18.0 | 0.833 |
| 11 | 2 | 53 | 13 | 2.0 | 17.2 | 0.749 |
| 9 (C) | 1 | 35 | 13 | 2.0 | 20.6 | 1.060 |
| 12 | 2 | 35 | 5 | 2.0 | 19.2 | 0.946 |
| 7 | 1 | 45 | 17 | 1.5 | 23.0 | 1.184 |
| 2 | 1 | 25 | 8 | 2.5 | 17.2 | 0.948 |
| 18 (C) | 2 | 35 | 13 | 2.0 | 18.0 | 0.952 |
| 13 | 2 | 35 | 21 | 2.0 | 19.4 | 0.887 |
| 20 (C) | 2 | 35 | 13 | 2.0 | 20.3 | 0.946 |
| 5 | 1 | 45 | 8 | 1.5 | 23.0 | 1.184 |
| 15 | 2 | 35 | 13 | 2.9 | 16.0 | 0.764 |
| 17 (C) | 1 | 35 | 13 | 2.0 | 22.0 | 1.522 |
| 6 | 1 | 45 | 8 | 2.5 | 17.0 | 0.752 |
| 14 | 2 | 35 | 13 | 1.1 | 14.0 | 0.474 |
| 10 | 2 | 17 | 13 | 2.0 | 17.0 | 0.617 |
| 16 (C) | 2 | 35 | 13 | 2.0 | 19.4 | 1.089 |
| 19 (C) | 1 | 35 | 13 | 2.0 | 19.8 | 1.163 |
| Compound | Traditional (μg/mL) | UAE (μg/mL) | Significance |
|---|---|---|---|
| Gallic acid | 124 ± 19 | 100 ± 14 | p = 0.1555 |
| Procyanidin B1 | 18.7 ± 1.5 | 20.0 ± 1.0 | p = 0.0788 |
| Procyanidin B2 | 2.70 ± 0.08 | 4.8 ± 0.6 | p = 0.0049 |
| Epicatechin | 1.11 ± 0.19 | 0.67 ± 0.02 | p = 0.0153 |
| Chlorogenic acid | 0.20 ± 0.03 | 0.26 ± 0.04 | p = 0.1423 |
| 4-hydroxycoumaric acid | 1.87 ± 0.03 | 1.76 ± 0.08 | p = 0.0481 |
| Ferulic acid | 3.35 ± 0.08 | 1.83 ± 0.09 | p = 0.0026 |
| Myricitrin | 1.3 ± 0.4 | 2.1 ± 0.4 | p = 0.0436 |
| Quercitrin | 2.1 ± 0.6 | 7.1 ± 0.8 | p = 0.0011 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clodoveo, M.L.; Crupi, P.; Muraglia, M.; Corbo, F. Processing of Carob Kernels to Syrup by Ultrasound-Assisted Extraction. Processes 2022, 10, 983. https://0-doi-org.brum.beds.ac.uk/10.3390/pr10050983
Clodoveo ML, Crupi P, Muraglia M, Corbo F. Processing of Carob Kernels to Syrup by Ultrasound-Assisted Extraction. Processes. 2022; 10(5):983. https://0-doi-org.brum.beds.ac.uk/10.3390/pr10050983
Chicago/Turabian StyleClodoveo, Maria Lisa, Pasquale Crupi, Marilena Muraglia, and Filomena Corbo. 2022. "Processing of Carob Kernels to Syrup by Ultrasound-Assisted Extraction" Processes 10, no. 5: 983. https://0-doi-org.brum.beds.ac.uk/10.3390/pr10050983