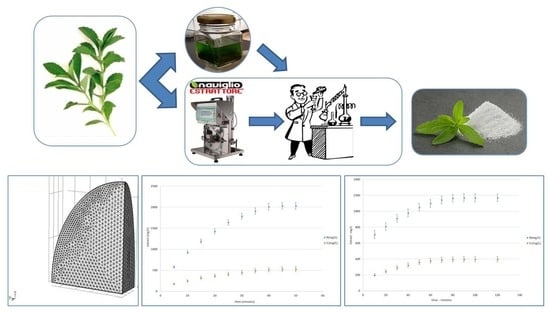
Comparison between Two Solid-Liquid Extraction Methods for the Recovery of Steviol Glycosides from Dried Stevia Leaves Applying a Numerical Approach
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composition of the Stevia “Benevento” Leaves
2.3. Extraction Process for the Stevia Solid Matrix
2.4. HPLC Analysis
2.5. Determination of the Component Size of the Stevia Solid Matrix
2.6. Determination of the Total Volume
2.7. Determination of the Initial Concentration of Stevia Solid Matrix Component
2.8. Rapid Solid-Liquid Dynamic Extraction Procedure (I)
2.9. Determination of the Dry Residue of the Extract at 105 °C
2.10. Analytical Approach
2.11. Finite Element Approach for the Cyclically Pressurized Extraction Process
- The diffusion values are independent of the value of the concentration.
- The considered process is assumed as isothermal; therefore, the thermal exchanges are neglected.
- The stevia solid matrix is homogeneous and isotropic.
- During the considered process, the matrix surface maintains the saturation concentration value (boundary condition).
- The solute content initial value for the stevia solid matrix had a uniform distribution and was constant within the solid matrices.
- (1)
- The effective diffusion coefficient of the moisture in the grain for a spherical geometry of the initial essential oil content of the grain;
- (2)
- The equilibrium essential oil content of the grain;
- (3)
- The total experimental time of the oil extraction.
- (1)
- Diffusivity coefficient (m2/s) for each component R and S;
- (2)
- Uniform initial value (mol/m3), where the relative activities of each fraction were determined on the basis of molar concentration (mol/L) or mass concentration (g/L);
- (3)
- Mass flow that was withdrawn by means of a kinetics curve.
3. Results and Discussion
3.1. Steviol Glycosides Analysis
3.2. Determination of the Kinetics of Steviol Glycoside Extraction from Stevia Solid Matrix in Hot Batches
3.3. Kinetics of the Cyclically Pressurized Cold Extraction (I)
3.4. Rapid Solid-Liquid Dynamic Extraction Process (I)
- (1)
- L1 length along the X-axis of the considered component (largest principal dimension, mm);
- (2)
- L2 length along the Y-axis of the considered component (second largest principal dimension, mm);
- (3)
- L3 length along the Z-axis of the considered component (smallest principal dimension, mm).
3.5. Numerical Analysis Data
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Savita, S.M.; Sheela, K.; Sunanda, S.; Shankar, A.G.; Ramakrishna, P. Stevia rebaudiana—A functional component for food industry. J. Hum. Ecol. 2004, 15, 261–264. [Google Scholar] [CrossRef]
- Serio, L. La Stevia rebaudiana, une alternative au sucre. Phytothérapie 2010, 8, 26–32. [Google Scholar] [CrossRef]
- Liu, S.; Manson, J.E. Dietary carbohydrates, physical inactivity, obesity, and the “metabolic syndrome” as predictors of coronary heart disease. Curr. Opin. Lipidol. 2001, 12, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, H. (Ed.) Sweeteners and Sugar Alternatives in Food Technology; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Pereira, C.; Storck, L.; Lopes, S.J.; Martin, T.N.; Bisognin, D.A. Dry biomass and glycosides yield from Stevia rebaudiana leaves under different harvesting times. Biosci. J. 2016, 32. [Google Scholar] [CrossRef]
- Abou-Arab, A.E.; Abou-Arab, A.A.; Abu-Salem, M.F. Physico-chemical assessment of natural sweeteners steviosides produced from Stevia rebaudiana Bertoni plant. Afr. J. Food Sci. 2010, 4, 269–281. [Google Scholar]
- Lemus-Mondaca, R.; Vega-Gálvez, A.; Zura-Bravo, L.; Ah-Hen, K. Stevia rebaudiana Bertoni, source of a high-potency natural sweetener: A comprehensive review on the biochemical, nutritional and functional aspects. Food Chem. 2012, 132, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Tateo, F.; Sanchez, M.; Escobar, L.; Bononi, M.; Lubian, E. Stevioside content of Stevia rebaudiana (Bertoni) Bertoni grown in east Paraguay. Ital. J. Food Sci. 1999, 11, 265–269. [Google Scholar]
- Jaitak, V.; Bandna, B.S.; Kaul, V.K. An efficient microwave-assisted extraction process of stevoiside and rebaudioside A from Stevia rebaudiana (Bertoni). Phytochem. Anal. 2009, 20, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Fu, J.-F.; Di, D.-L. Preparative isolation and purificationof steviol glycosides from Stevia rebaudiana Bertoni using high-speed counter-corrent chromatography. Sep. Purif. Technol. 2010, 71, 220–224. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J.; Tiwary, A.K. Optimisation of novel method for the extraction of steviosides from Stevia rebaudiana leaves. Food Chem. 2012, 132, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Woelwer-Rieck, U.; Lankes, C.; Wawrzun, A.; Wüst, M. Improved HPLC method for the evaluation of the major steviol glycosides in leaves of Stevia rebaudiana. Eur. Food Res. Technol. 2010, 231, 581–588. [Google Scholar] [CrossRef]
- Martins, P.M.; Thorat, B.N.; Lanchote, A.D.; Freitas, L.A. Green extraction of glycosides from Stevia rebaudiana (Bert.) with low solvent consumption: A desirability approach. Resour.-Effic. Technol. 2016, 2, 247–253. [Google Scholar] [CrossRef]
- Martins, P.M.; Lanchote, A.D.; Thorat, B.N.; Freitas, L.A. Turbo-extraction of glycosides from Stevia rebaudiana using a fractional factorial design. Rev. Bras. Farmacogn. 2017, 27, 510–518. [Google Scholar] [CrossRef]
- Kovačević, D.B.; Barba, F.J.; Granato, D.; Galanakis, C.M.; Herceg, Z.; Dragović-Uzelac, V.; Putnik, P. Pressurized hot water extraction (PHWE) for the green recovery of bioactive compounds and steviol glycosides from Stevia rebaudiana Bertoni leaves. Food Chem. 2018, 254, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Bridel, M.; Lavieille, R. Le principe à saveur sucrée du Kaà-hê-é (Stevia rebaudiana) Bertoni. J. Pharm. Clin 1931, 14, 99–154. [Google Scholar]
- Mosettig, E.; Beglinger, U.; Dolder, F.; Lichti, H.; Quitt, P.; Waters, J.A. The absolute configuration of steviol and isosteviol. J. Am. Chem. Soc. 1963, 85, 2305–2309. [Google Scholar] [CrossRef]
- Wölwer-Rieck, U.; Tomberg, W.; Wawrzun, A. Investigations on the stability of stevioside and rebaudioside A in soft drinks. J. Agric. Food Chem. 2010, 58, 12216–12220. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, P.B.; Gregersen, S.; Poulsen, C.R.; Hermansen, K. Stevioside acts directly on pancreatic beta cells to secrete insulin: actions independent of cyclic adenosine monophosphate and adenosine triphosphate-sensitive K+-channel activity. Metabolism 2000, 9, 208–214. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, K.R.; Park, J.R.; Kim, K.S.; Tchai, B.S. A study on the safety of the stevioside as a new sweetening source. Korean J. Food Sci. Technol. 1979, 11, 224–231. [Google Scholar]
- Geuns, J.M.C. Safety evaluation of Stevia and stevioside. Stud. Nat. Prod. Chem. 2002, 27, 299–319. [Google Scholar]
- Geuns, J.M.; Augustijns, P.; Mols, R.; Buyse, J.G.; Driessen, B. Metabolism of stevioside in pigs and intestinal absorption characteristics of stevioside, rebaudioside A and steviol. Food Chem. Toxicol. 2003, 41, 1599–1607. [Google Scholar] [CrossRef]
- Dozono, F. U.S. Patent No. 5262161, 1993. (1993-11-16). Available online: http: //www.chemweb.com/ databases /patents (accessed on 30 July 2018).
- Takahashi, K.; Matsuda, M.; Ohashi, K.; Taniguchi, K.; Nakagomi, O.; Abe, Y.; Mori, S.; Sato, N.; Shigeta, S. Analysis of anti-rotavirus of extract from Stevia rebaudiana. Antivir. Res. 2001, 49, 15–24. [Google Scholar] [CrossRef]
- COMMISSION REGULATION (EU) No 1131/2011, 2011. (OJ L 295, p205, 12/11/2011) of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council with regard to steviol glycosides. Available online: https://www.fsai.ie/uploadedFiles/Reg1131_2011.pdf (accessed on 30 July 2018).
- Gallo, M.; Vitulano, M.; Andolfi, A.; DellaGreca, M.; Naviglio, D. Rapid solid-liquid dynamic extraction (RSLDE), a new rapid and greener method to extract two steviol glycosides (stevioside and rebaudioside A) from Stevia leaves. Plant Foods Hum. Nutr. 2017, 72, 141–148. [Google Scholar] [CrossRef] [PubMed]
- American Society of Agriculture Engineers (ASAE). ASAE Standard S352.2: Moisture Measurement—Unground Grain and Seeds; ASAE: St. Joseph, MI, USA, 1997. [Google Scholar]
- Jacobes, M.B. The Chemical Analysis of Foods and Food Products; Van Nostrand Reinhold Company: New York, NY, USA; Cincinnati, OH, USA; Toronto, ON, Canada; London, UK; Melbourne, Australia, 2013. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of the Association of Official Analytical Chemists; Williams, S. Publisher: Arlington, VA, USA, 2016. [Google Scholar]
- Ceunen, S.; Geuns, J.M. Steviol glycosides: chemical diversity, metabolism, and function. J. Nat. Prod. 2013, 76, 1201–1228. [Google Scholar] [CrossRef] [PubMed]
- Vanek, T.; Nevopim, A.; Valicek, P. Determination of Stevioside in plant material and fruit teas. J. Food Compos. Anal. 2001, 14, 383–388. [Google Scholar] [CrossRef]
- Kedik, S.A.; Fedorov, S.V.; Yanul’, N.A.; Prokhorova, L.V.; Smirnova, E.V.; Panov, A.V. Chromatographic determination of stevioside in raw plant material. Pharm. Chem. J. 2003, 37, 529–532. [Google Scholar] [CrossRef]
- Jain, R.K.; Bal, S. Properties of pearl millet. J. Agric. Eng. Res. 1997, 66, 85–91. [Google Scholar] [CrossRef]
- Carillo, M.; Formato, A.; Fabiani, A.; Scaglione, G.; Pucillo, G.P. An inertizing and cooling process for grapes cryomaceration. Electron. J. Biotechnol. 2011, 14. [Google Scholar] [CrossRef]
- Formato, A.; Naviglio, D.; Pucillo, G.P.; Nota, G. Improved fumigating process for stored foodstuffs by phosphine in sealed chambers. J. Agric. Food Chem. 2012, 60, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Formato, A.; Gallo, M.; Ianniello, D.; Montesano, D.; Naviglio, D. Supercritical fluid extraction of alpha- and beta-acids from hops compared to cyclically pressurized solid-liquid extraction. J. Supercrit. Fluids 2013, 84, 113–120. [Google Scholar] [CrossRef]
- Gallo, M.; Formato, A.; Ianniello, D.; Andolfi, A.; Conte, E.; Ciaravolo, M.; Varchetta, V.; Naviglio, D. Supercritical fluid extraction of pyrethrins from pyrethrum flowers (Chrysanthemum cinerariifolium) compared to traditional maceration and cyclic pressurization extraction. J. Supercrit. Fluids 2017b, 119, 104–112. [Google Scholar] [CrossRef]
- Naviglio, D.; Formato, A.; Pucillo, G.P.; Gallo, M. Cyclically pressurized soaking process for hydration and aromatization of cannellini beans. J. Food Eng. 2013, 116, 765–774. [Google Scholar] [CrossRef]
- Naviglio, D.; Formato, A.; Gallo, M. Comparison between 2 methods of solid-liquid extraction for the production of Cinchona calisaya elixir: An experimental kinetics and numerical modeling approach. J. Food Sci. 2014, 79, E1704–E1712. [Google Scholar] [CrossRef] [PubMed]
- Naviglio, D.; Formato, A.; Vitulano, M.; Cozzolino, I.; Ferrara, L.; Zanoelo, E.F.; Gallo, M. Comparison between the kinetics of conventional maceration and a cyclic pressurization extraction process for the production of lemon liqueur using a numerical model. J. Food Process Eng. 2017. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Oxford University Press: New York, NY, USA, 1975. [Google Scholar]
- Gaston, A.L.; Abalone, R.M.; Giner, S.A.; Bruce, D.M. Effect of modelling assumptions on the effective water diffusivity in wheat. Biosyst. Eng. 2004, 88, 175–185. [Google Scholar] [CrossRef]
- Gaston, A.L.; Abalone, R.M.; Giner, S.A. Wheat drying kinetics. Diffusivities for sphere and ellipsoid by finite elements. J. Food Eng. 2002, 52, 313–322. [Google Scholar] [CrossRef]
- McCabe, W.L.; Smith, J.C.; Harriott, P. Unit Operation of Chemical Engineering; McGraw Hill Book Company: New York, NY, USA, 1993. [Google Scholar]
- Ahromrit, A.; Ledward, D.A.; Niranjan, K. 2006. High pressure induced water uptake characteristics of Thai glutinous rice. J. Food Eng. 2006, 72, 225–233. [Google Scholar] [CrossRef]
- Hsu, K.H.; Kim, C.J.; Wilson, L.A. Factors affecting water uptake of soybeans during soaking. Cereal Chem. 1983, 60, 208–211. [Google Scholar]
- Plhak, L.C.; Caldwell, K.B.; Stanley, D.W. Comparison of methods used to characterize water inhibition in hard-to-cook beans. J. Food Sci. 1989, 54, 326–329. [Google Scholar] [CrossRef]





| Time (min) | L1 Length (mm) | L2 Width (mm) | L3 Thickness (mm) | Geometric Mean Diameter (GMD) = D (mm) | Sphericity | Sphericity Factor (fe) |
|---|---|---|---|---|---|---|
| t0 = 0 | 7.05 ± 1.81 | 5.22 ± 1.51 | 1.51 ± 1.21 | 2.80 | 0.860 | 0.519 |
| t1 = 10 | 7.01 ± 1.78 | 5.01 ± 1.55 | 1.49 ± 1.19 | 2.73 | 0.856 | 0.510 |
| t1 = 20 | 6.93 ± 1.78 | 4.98 ± 1.55 | 1.46 ± 1.19 | 2.69 | 0.855 | 0.501 |
| t1 = 30 | 6.90 ± 1.78 | 4.95 ± 1.55 | 1.43 ± 1.19 | 2.66 | 0.853 | 0.490 |
| t1 = 40 | 6.86 ± 1.78 | 4.93 ± 1.55 | 1.40 ± 1.19 | 2.62 | 0.852 | 0.460 |
| t1 = 50 | 6.84 ± 1.78 | 4.90 ± 1.55 | 1.36 ± 1.19 | 2.58 | 0.849 | 0.435 |
| t1 = 60 | 6.81 ± 1.78 | 4.86 ± 1.55 | 1.33 ± 1.19 | 2.54 | 0.846 | 0.421 |
| t1 = 70 | 6.78 ± 1.78 | 4.83 ± 1.55 | 1.29 ± 1.19 | 2.49 | 0.843 | 0.749 |
| t1 = 80 | 6.75 ± 1.78 | 4.80 ± 1.55 | 1.26 ± 1.19 | 2.45 | 0.841 | 0.744 |
| t2 = 90 | 6.71 ± 1.69 | 4.77 ± 1.36 | 1.22 ± 1.13 | 2.41 | 0.838 | 0.401 |
| t2 = 100 | 6.67 ± 1.69 | 4.74 ± 1.36 | 1.19 ± 1.13 | 2.37 | 0.836 | 0.730 |
| t2 = 120 | 6.64 ± 1.68 | 4.70 ± 1.24 | 1.16 ± 1.12 | 2.33 | 0.833 | 0.395 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, M.; Formato, A.; Formato, G.; Naviglio, D. Comparison between Two Solid-Liquid Extraction Methods for the Recovery of Steviol Glycosides from Dried Stevia Leaves Applying a Numerical Approach. Processes 2018, 6, 105. https://0-doi-org.brum.beds.ac.uk/10.3390/pr6080105
Gallo M, Formato A, Formato G, Naviglio D. Comparison between Two Solid-Liquid Extraction Methods for the Recovery of Steviol Glycosides from Dried Stevia Leaves Applying a Numerical Approach. Processes. 2018; 6(8):105. https://0-doi-org.brum.beds.ac.uk/10.3390/pr6080105
Chicago/Turabian StyleGallo, Monica, Andrea Formato, Gaetano Formato, and Daniele Naviglio. 2018. "Comparison between Two Solid-Liquid Extraction Methods for the Recovery of Steviol Glycosides from Dried Stevia Leaves Applying a Numerical Approach" Processes 6, no. 8: 105. https://0-doi-org.brum.beds.ac.uk/10.3390/pr6080105