Anaerobic Co-Digestion of Wastewater Sludge: A Review of Potential Co-Substrates and Operating Factors for Improved Methane Yield
Abstract
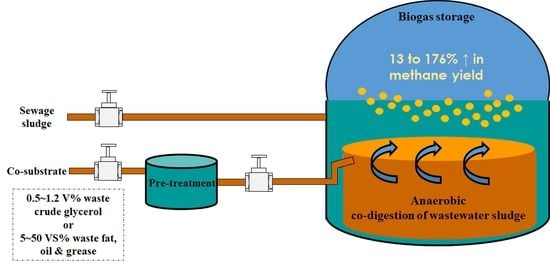
:1. Introduction
2. Potential Co-Substrates for Anaerobic Co-Digestion of Wastewater Sludge
2.1. Organic Fraction of Municipal Solid Waste (OFMSW) and Food Waste (FW)
2.2. Crude Glycerol
2.3. Agricultural Wastes
2.4. Fat, Oil and Grease (FOG)
3. Factors Influencing Co-Digestion Performance
3.1. Mixing
3.2. Co-Substrate Mixing Ratio and Nutrient Balance
3.3. Operating Temperature
3.4. Organic Loading Rate (OLR) and Hydraulic Retention Time (HRT)
4. Summary and Challenges of ACD of Wastewater Sludge
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chan, Y.J.; Chong, M.F.; Law, C.L. Performance and kinetic evaluation of an integrated anaerobic–aerobic bioreactor in the treatment of palm oil mill effluent. Environ. Technol. 2017, 38, 1005–1021. [Google Scholar] [CrossRef] [PubMed]
- Musa, N.S.; Ahmad, W.A. Chemical oxygen demand reduction in industrial wastewater using locally isolated bacteria. Malays. J. Fundam. Appl. Sci. 2010, 6. [Google Scholar] [CrossRef]
- Braun, R.; Wellinger, A. Potential of Co-Digestion; IEA: Paris, France, 2002. [Google Scholar]
- Mata-Alvarez, J.; Mace, S.; Llabres, P. Anaerobic digestion of organic solid wastes. An overview of research achievements and perspectives. Bioresour. Technol. 2000, 74, 3–16. [Google Scholar] [CrossRef]
- Long, J.H.; Aziz, T.N.; Francis III, L.; Ducoste, J.J. Anaerobic co-digestion of fat, oil, and grease (FOG): A review of gas production and process limitations. Process Saf. Environ. Prot. 2012, 90, 231–245. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Surendra, K.; Takara, D.; Oechsner, H.; Khanal, S.K. Anaerobic digestion of lignocellulosic biomass: Challenges and opportunities. Bioresour. Technol. 2015, 178, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Zhou, Q.; Fu, G.; Li, Y. Semi-continuous anaerobic co-digestion of thickened waste activated sludge and fat, oil and grease. Waste Manag. 2011, 31, 1752–1758. [Google Scholar] [CrossRef] [PubMed]
- Astals, S.; Nolla-Ardèvol, V.; Mata-Alvarez, J. Anaerobic co-digestion of pig manure and crude glycerol at mesophilic conditions: Biogas and digestate. Bioresour. Technol. 2012, 110, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Wu, C.; Li, Y.; Li, W.; Wang, K. Characterizing the distribution of organic matter during composting of sewage sludge using a chemical and spectroscopic approach. RSC Adv. 2015, 5, 95960–95966. [Google Scholar] [CrossRef]
- Hallaji, S.M.; Kuroshkarim, M.; Moussavi, S.P. Enhancing methane production using anaerobic co-digestion of waste activated sludge with combined fruit waste and cheese whey. BMC Biotechnol. 2019, 19, 19. [Google Scholar] [CrossRef]
- Rabii, A.; Aldin, S.; Dahman, Y.; Elbeshbishy, E. A review on anaerobic co-digestion with a focus on the microbial populations and the effect of multi-stage digester configuration. Energies 2019, 12, 1106. [Google Scholar] [CrossRef] [Green Version]
- Campuzano, R.; González-Martínez, S. Characteristics of the organic fraction of municipal solid waste and methane production: A review. Waste Manag. 2016, 54, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Duan, N.; Dong, B.; Dai, L. High-solids anaerobic co-digestion of sewage sludge and food waste in comparison with mono digestions: Stability and performance. Waste Manag. 2013, 33, 308–316. [Google Scholar] [CrossRef]
- Chow, W.L.; Chan, Y.J.; Chong, M.F. A new energy source from the anaerobic co-digestion (acd) treatment of oleo chemical effluent with glycerin pitch. Asia Pac. J. Chem. Eng. 2015, 10, 556–564. [Google Scholar] [CrossRef]
- EPA. Municipal Solid Waste; United States Environmental Protection Agency (EPA): Washington, DC, USA, 1996.
- Paritosh, K.; Yadav, M.; Mathur, S.; Balan, V.; Liao, W.; Pareek, N.; Vivekanand, V. Organic fraction of municipal solid waste: Overview of treatment methodologies to enhance anaerobic biodegradability. Front. Energy Res. 2018, 6, 75. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.Y.; Chiu, S.L.; Lo, I.M. Effects of moisture content of food waste on residue separation, larval growth and larval survival in black soldier fly bioconversion. Waste Manag. 2017, 67, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Cabbai, V.; Ballico, M.; Aneggi, E.; Goi, D. BMP tests of source selected OFMSW to evaluate anaerobic codigestion with sewage sludge. Waste Manag. 2013, 33, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Duan, N.; Dong, B.; Wu, B.; Dai, X. High-solid anaerobic digestion of sewage sludge under mesophilic conditions: Feasibility study. Bioresour. Technol. 2012, 104, 150–156. [Google Scholar] [CrossRef]
- Mendez, R.; Lema, J.M.; Soto, M. Treatment of seafood-processing wastewaters in mesophilic and thermophilic anaerobic filters. Water Environ. Res. 1995, 67, 33–45. [Google Scholar] [CrossRef]
- Silvestre, G.; Fernández, B.; Bonmatí, A. Addition of crude glycerine as strategy to balance the C/N ratio on sewage sludge thermophilic and mesophilic anaerobic co-digestion. Bioresour. Technol. 2015, 193, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, C.; Bolzonella, D.; Pavan, P.; Fatone, F.; Cecchi, F. Mesophilic and thermophilic anaerobic co-digestion of waste activated sludge and source sorted biowaste in pilot-and full-scale reactors. Renew. Energy 2013, 55, 260–265. [Google Scholar] [CrossRef]
- Al bkoor Alrawashdeh, K.; Pugliese, A.; Slopiecka, K.; Pistolesi, V.; Massoli, S.; Bartocci, P.; Bidini, G.; Fantozzi, F. Codigestion of untreated and treated sewage sludge with the organic fraction of municipal solid wastes. Fermentation 2017, 3, 35. [Google Scholar] [CrossRef] [Green Version]
- Macias-Corral, M.; Samani, Z.; Hanson, A.; Smith, G.; Funk, P.; Yu, H.; Longworth, J. Anaerobic digestion of municipal solid waste and agricultural waste and the effect of co-digestion with dairy cow manure. Bioresour. Technol. 2008, 99, 8288–8293. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jha, A.K.; He, J.; Ban, Q.; Chang, S.; Wang, P. Assessment of the effects of dry anaerobic co-digestion of cow dung with waste water sludge on biogas yield and biodegradability. Int. J. Phys. Sci. 2011, 6, 3679–3688. [Google Scholar]
- Silvestre, G.; Bonmatí, A.; Fernández, B. Optimisation of sewage sludge anaerobic digestion through co-digestion with OFMSW: Effect of collection system and particle size. Waste Manag. 2015, 43, 137–143. [Google Scholar] [CrossRef]
- Kim, H.-W.; Nam, J.-Y.; Shin, H.-S. A comparison study on the high-rate co-digestion of sewage sludge and food waste using a temperature-phased anaerobic sequencing batch reactor system. Bioresour. Technol. 2011, 102, 7272–7279. [Google Scholar] [CrossRef]
- Di Maria, F.; Micale, C.; Contini, S. Energetic and environmental sustainability of the co-digestion of sludge with bio-waste in a life cycle perspective. Appl. Energy 2016, 171, 67–76. [Google Scholar] [CrossRef]
- Ma, J.; Van Wambeke, M.; Carballa, M.; Verstraete, W. Improvement of the anaerobic treatment of potato processing wastewater in a UASB reactor by co-digestion with glycerol. Biotechnol. Lett. 2008, 30, 861–867. [Google Scholar] [CrossRef]
- Astals, S.; Nolla-Ardèvol, V.; Mata-Alvarez, J. Thermophilic co-digestion of pig manure and crude glycerol: Process performance and digestate stability. J. Biotechnol. 2013, 166, 97–104. [Google Scholar] [CrossRef]
- Knothe, G.; Krahl, J.; Gerpen, J.V. The Biodiesel Handbook, 2nd ed.; AOCS Press: Urbana, IL, USA, 2010. [Google Scholar]
- Anuar, M.R.; Abdullah, A.Z. Challenges in biodiesel industry with regards to feedstock, environmental, social and sustainability issues: A critical review. Renew. Sustain. Energy Rev. 2016, 58, 208–223. [Google Scholar] [CrossRef]
- Monteiro, M.R.; Kugelmeier, C.L.; Pinheiro, R.S.; Batalha, M.O.; da Silva César, A. Glycerol from biodiesel production: Technological paths for sustainability. Renew. Sustain. Energy Rev. 2018, 88, 109–122. [Google Scholar] [CrossRef]
- Pinheiro, R.S. Processos de Inovação Tecnológica Para a Glicerina Produzida no Processo de Obtenção de Biodiesel no Brasil; Universidade Federal de Sao Carlos: Sao Carlos, Brazil, 2011. [Google Scholar]
- Aguilar-Aguilar, F.A.; Nelson, D.L.; Pantoja, L.d.A.; Santos, A. Study of anaerobic co-digestion of crude glycerol and swine manure for the production of biogas. Rev. Virtual Quim 2017, 9, 2383–2403. [Google Scholar] [CrossRef]
- Fountoulakis, M.; Petousi, I.; Manios, T. Co-digestion of sewage sludge with glycerol to boost biogas production. Waste Manag. 2010, 30, 1849–1853. [Google Scholar] [CrossRef] [PubMed]
- Rivero, M.; Solera, R.; Perez, M. Anaerobic mesophilic co-digestion of sewage sludge with glycerol: Enhanced biohydrogen production. Int. J. Hydrog. Energy 2014, 39, 2481–2488. [Google Scholar] [CrossRef]
- Dos Santos Ferreira, J.; Volschan, I.; Cammarota, M.C. Co-digestion of sewage sludge with crude or pretreated glycerol to increase biogas production. Environ. Sci. Pollut. Res. 2018, 25, 21811–21821. [Google Scholar] [CrossRef] [PubMed]
- Razaviarani, V.; Buchanan, I.D.; Malik, S.; Katalambula, H. Pilot scale anaerobic co-digestion of municipal wastewater sludge with biodiesel waste glycerin. Bioresour. Technol. 2013, 133, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Nartker, S.; Ammerman, M.; Aurandt, J.; Stogsdil, M.; Hayden, O.; Antle, C. Increasing biogas production from sewage sludge anaerobic co-digestion process by adding crude glycerol from biodiesel industry. Waste Manag. 2014, 34, 2567–2571. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.; Astals, S.; Lu, Y.; Devadas, M.; Batstone, D. Anaerobic codigestion of sewage sludge and glycerol, focusing on process kinetics, microbial dynamics and sludge dewaterability. Water Res. 2014, 67, 355–366. [Google Scholar] [CrossRef]
- Obi, F.; Ugwuishiwu, B.; Nwakaire, J. Agricultural waste concept, generation, utilization and management. Niger. J. Technol. 2016, 35, 957–964. [Google Scholar] [CrossRef]
- MacLellan, J.; Chen, R.; Kraemer, R.; Zhong, Y.; Liu, Y.; Liao, W. Anaerobic treatment of lignocellulosic material to co-produce methane and digested fiber for ethanol biorefining. Bioresour. Technol. 2013, 130, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Teater, C.; MacLellan, J.; Liu, Y.; Liao, W. Development of a new bioethanol feedstock–anaerobically digested fiber from confined dairy operations using different digestion configurations. Biomass Bioenergy 2011, 35, 1946–1953. [Google Scholar] [CrossRef]
- Nayal, F.S.; Mammadov, A.; Ciliz, N. Environmental assessment of energy generation from agricultural and farm waste through anaerobic digestion. J. Environ. Manag. 2016, 184, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Wickham, R.; Nghiem, L.D. Synergistic effect from anaerobic co-digestion of sewage sludge and organic wastes. Int. Biodeterior. Biodegrad. 2017, 116, 191–197. [Google Scholar] [CrossRef]
- Maragkaki, A.; Fountoulakis, M.; Gypakis, A.; Kyriakou, A.; Lasaridi, K.; Manios, T. Pilot-scale anaerobic co-digestion of sewage sludge with agro-industrial by-products for increased biogas production of existing digesters at wastewater treatment plants. Waste Manag. 2017, 59, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Maragkaki, A.; Fountoulakis, M.; Kyriakou, A.; Lasaridi, K.; Manios, T. Boosting biogas production from sewage sludge by adding small amount of agro-industrial by-products and food waste residues. Waste Manag. 2018, 71, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Alagöz, B.A.; Yenigün, O.; Erdinçler, A. Ultrasound assisted biogas production from co-digestion of wastewater sludges and agricultural wastes: Comparison with microwave pre-treatment. Ultrason. Sonochem. 2018, 40, 193–200. [Google Scholar] [CrossRef]
- Szaja, A.; Montusiewicz, A. Enhancing the co-digestion efficiency of sewage sludge and cheese whey using brewery spent grain as an additional substrate. Bioresour. Technol. 2019, 291, 121863. [Google Scholar] [CrossRef]
- Montañés, R.; Pérez, M.; Solera, R. Mesophilic anaerobic co-digestion of sewage sludge and a lixiviation of sugar beet pulp: Optimisation of the semi-continuous process. Bioresour. Technol. 2013, 142, 655–662. [Google Scholar] [CrossRef]
- Alagöz, B.A.; Yenigün, O.; Erdinçler, A. Enhancement of anaerobic digestion efficiency of wastewater sludge and olive waste: Synergistic effect of co-digestion and ultrasonic/microwave sludge pre-treatment. Waste Manag. 2015, 46, 182–188. [Google Scholar] [CrossRef]
- Salama, E.-S.; Saha, S.; Kurade, M.B.; Dev, S.; Chang, S.W.; Jeon, B.-H. Recent trends in anaerobic co-digestion: Fat, oil, and grease (FOG) for enhanced biomethanation. Prog. Energy Combust. Sci. 2019, 70, 22–42. [Google Scholar] [CrossRef]
- Fonda, K.D.; Hetherington, M.; Kawamoto, M.H. Dealing with FOG: A problem or an opportunity. Proc. Water Environ. Fed. 2003, 2003, 654–678. [Google Scholar] [CrossRef]
- Kabouris, J.C.; Tezel, U.; Pavlostathis, S.G.; Engelmann, M.; Todd, A.C.; Gillette, R.A. The anaerobic biodegradability of municipal sludge and fat, oil, and grease at mesophilic conditions. Water Environ. Res. 2008, 80, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Masse, L.; Masse, D.; Kennedy, K.; Chou, S. Neutral fat hydrolysis and long-chain fatty acid oxidation during anaerobic digestion of slaughterhouse wastewater. Biotechnol. Bioeng. 2002, 79, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Cavaleiro, A.J.; Salvador, A.F.; Alves, J.I.; Alves, M. Continuous high rate anaerobic treatment of oleic acid based wastewater is possible after a step feeding start-up. Environ. Sci. Technol. 2009, 43, 2931–2936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madigan, M.T.; Martinko, J.M.; Parker, J.; Brock, T.D. Brock Biology of Microorganisms, 11th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2006. [Google Scholar]
- Pavlostathis, S.; Giraldo-Gomez, E. Kinetics of anaerobic treatment: A critical review. Crit. Rev. Environ. Sci. Technol. 1991, 21, 411–490. [Google Scholar] [CrossRef]
- Canakci, M.; Van Gerpen, J. Biodiesel production from oils and fats with high free fatty acids. Trans. ASAE 2001, 44, 1429. [Google Scholar] [CrossRef]
- Jeganathan, J.; Nakhla, G.; Bassi, A. Long-term performance of high-rate anaerobic reactors for the treatment of oily wastewater. Environ. Sci. Technol. 2006, 40, 6466–6472. [Google Scholar] [CrossRef]
- Luostarinen, S.; Luste, S.; Sillanpää, M. Increased biogas production at wastewater treatment plants through co-digestion of sewage sludge with grease trap sludge from a meat processing plant. Bioresour. Technol. 2009, 100, 79–85. [Google Scholar] [CrossRef]
- Girault, R.; Bridoux, G.; Nauleau, F.; Poullain, C.; Buffet, J.; Peu, P.; Sadowski, A.; Béline, F. Anaerobic co-digestion of waste activated sludge and greasy sludge from flotation process: Batch versus CSTR experiments to investigate optimal design. Bioresour. Technol. 2012, 105, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Pereira, M.; Pires, O.; Mota, M.; Alves, M. Anaerobic biodegradation of oleic and palmitic acids: Evidence of mass transfer limitations caused by long chain fatty acid accumulation onto the anaerobic sludge. Biotechnol. Bioeng. 2005, 92, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Rinzema, A. Anaerobic Treatment of Wastewater with High Concentrations of Lipids or Sulfate; Staff: Wageningen, The Netherlands, 1988. [Google Scholar]
- Kuang, Y. Enhancing Anaerobic Degradation of Lipids in Wastewater by Addition of Co-Substrate; Murdoch University: Perth, Australia, 2002. [Google Scholar]
- Davidsson, Å.; Lövstedt, C.; la Cour Jansen, J.; Gruvberger, C.; Aspegren, H. Co-digestion of grease trap sludge and sewage sludge. Waste Manag. 2008, 28, 986–992. [Google Scholar] [CrossRef]
- Kabouris, J.C.; Tezel, U.; Pavlostathis, S.G.; Engelmann, M.; Dulaney, J.A.; Todd, A.C.; Gillette, R.A. Mesophilic and thermophilic anaerobic digestion of municipal sludge and fat, oil, and grease. Water Environ. Res. 2009, 81, 476–485. [Google Scholar] [CrossRef]
- Silvestre, G.; Rodríguez-Abalde, A.; Fernández, B.; Flotats, X.; Bonmatí, A. Biomass adaptation over anaerobic co-digestion of sewage sludge and trapped grease waste. Bioresour. Technol. 2011, 102, 6830–6836. [Google Scholar] [CrossRef]
- Silvestre, G.; Illa, J.; Fernández, B.; Bonmatí, A. Thermophilic anaerobic co-digestion of sewage sludge with grease waste: Effect of long chain fatty acids in the methane yield and its dewatering properties. Appl. Energy 2014, 117, 87–94. [Google Scholar] [CrossRef]
- Alqaralleh, R.M.; Kennedy, K.; Delatolla, R.; Sartaj, M. Thermophilic and hyper-thermophilic co-digestion of waste activated sludge and fat, oil and grease: Evaluating and modeling methane production. J. Environ. Manag. 2016, 183, 551–561. [Google Scholar] [CrossRef]
- Grady, C.L., Jr.; Daigger, G.T.; Love, N.G.; Filipe, C.D. Biological Wastewater Treatment, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Stafford, D. The effects of mixing and volatile fatty acid concentrations on anaerobic digester performance. Biomass 1982, 2, 43–55. [Google Scholar] [CrossRef]
- Karim, K.; Hoffmann, R.; Klasson, K.T.; Al-Dahhan, M. Anaerobic digestion of animal waste: Effect of mode of mixing. Water Res. 2005, 39, 3597–3606. [Google Scholar] [CrossRef]
- Rizk, M.C.; Bergamasco, R.; Tavares, C.R.G. Anaerobic co-digestion of fruit and vegetable waste and sewage sludge. Int. J. Chem. React. Eng. 2007, 5. [Google Scholar] [CrossRef]
- Stroot, P.G.; McMahon, K.D.; Mackie, R.I.; Raskin, L. Anaerobic codigestion of municipal solid waste and biosolids under various mixing conditions—I. Digester performance. Water Res. 2001, 35, 1804–1816. [Google Scholar] [CrossRef]
- Latha, K.; Velraj, R.; Shanmugam, P.; Sivanesan, S. Mixing strategies of high solids anaerobic co-digestion using food waste with sewage sludge for enhanced biogas production. J. Clean. Prod. 2019, 210, 388–400. [Google Scholar] [CrossRef]
- Wang, M.; Sun, X.; Li, P.; Yin, L.; Liu, D.; Zhang, Y.; Li, W.; Zheng, G. A novel alternate feeding mode for semi-continuous anaerobic co-digestion of food waste with chicken manure. Bioresour. Technol. 2014, 164, 309–314. [Google Scholar] [CrossRef]
- Mahmoodi-Eshkaftaki, M.; Ebrahimi, R. Assess a new strategy and develop a new mixer to improve anaerobic microbial activities and clean biogas production. J. Clean. Prod. 2019, 206, 797–807. [Google Scholar] [CrossRef]
- Monnet, F. An introduction to anaerobic digestion of organic wastes. Remade Scotl. 2003, 379, 1–48. [Google Scholar]
- Heo, N.H.; Park, S.C.; Kang, H. Effects of mixture ratio and hydraulic retention time on single-stage anaerobic co-digestion of food waste and waste activated sludge. J. Environ. Sci. Health Part A 2004, 39, 1739–1756. [Google Scholar] [CrossRef]
- Hagos, K.; Zong, J.; Li, D.; Liu, C.; Lu, X. Anaerobic co-digestion process for biogas production: Progress, challenges and perspectives. Renew. Sustain. Energy Rev. 2017, 76, 1485–1496. [Google Scholar] [CrossRef]
- Siddique, M.N.I.; Wahid, Z.A. Achievements and perspectives of anaerobic co-digestion: A review. J. Clean. Prod. 2018, 194, 359–371. [Google Scholar] [CrossRef]
- Khalid, A.; Arshad, M.; Anjum, M.; Mahmood, T.; Dawson, L. The anaerobic digestion of solid organic waste. Waste Manag. 2011, 31, 1737–1744. [Google Scholar] [CrossRef]
- Li, Y.; Sasaki, H.; Yamashita, K.; Seki, K.; Kamigochi, I. High-rate methane fermentation of lipid-rich food wastes by a high-solids co-digestion process. Water Sci. Technol. 2002, 45, 143–150. [Google Scholar] [CrossRef]
- Lee, M.; Hidaka, T.; Hagiwara, W.; Tsuno, H. Comparative performance and microbial diversity of hyperthermophilic and thermophilic co-digestion of kitchen garbage and excess sludge. Bioresour. Technol. 2009, 100, 578–585. [Google Scholar] [CrossRef]
- Hansen, K.H.; Angelidaki, I.; Ahring, B.K. Anaerobic digestion of swine manure: Inhibition by ammonia. Water Res. 1998, 32, 5–12. [Google Scholar] [CrossRef]
- Fountoulakis, M.; Manios, T. Enhanced methane and hydrogen production from municipal solid waste and agro-industrial by-products co-digested with crude glycerol. Bioresour. Technol. 2009, 100, 3043–3047. [Google Scholar] [CrossRef]
- Vindis, P.; Mursec, B.; Janzekovic, M.; Cus, F. The impact of mesophilic and thermophilic anaerobic digestion on biogas production. J. Achiev. Mater. Manuf. Eng. 2009, 36, 192–198. [Google Scholar]
- Ghatak, M.; Mahanata, P. Effect of Temperature on Biogas Production from Rice Straw and Rice Husk. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Zeeman, G.; Wiegant, W.; Koster-Treffers, M.; Lettinga, G. The influence of the total-ammonia concentration on the thermophilic digestion of cow manure. Agric. Wastes 1985, 14, 19–35. [Google Scholar] [CrossRef]
- Hanaki, K.; Matsuo, T.; Nagase, M. Mechanism of inhibition caused by long-chain fatty acids in anaerobic digestion process. Biotechnol. Bioeng. 1981, 23, 1591–1610. [Google Scholar] [CrossRef]
- Hwu, C.-S.; Lettinga, G. Acute toxicity of oleate to acetate-utilizing methanogens in mesophilic and thermophilic anaerobic sludges. Enzym. Microb. Technol. 1997, 21, 297–301. [Google Scholar] [CrossRef]
- Kim, S.-H.; Han, S.-K.; Shin, H.-S. Feasibility of biohydrogen production by anaerobic co-digestion of food waste and sewage sludge. Int. J. Hydrog. Energy 2004, 29, 1607–1616. [Google Scholar] [CrossRef]
- Babaee, A.; Shayegan, J. Effect of organic loading rates (OLR) on production of methane from anaerobic digestion of vegetables waste. In Proceedings of the World Renewable Energy Congress-Sweden, Linkoping, Sweden, 8–13 May 2011; pp. 411–417. [Google Scholar]
- Mata-Alvarez, J.; Llabrés, P.; Cecchi, F.; Pavan, P. Anaerobic digestion of the Barcelona central food market organic wastes: Experimental study. Bioresour. Technol. 1992, 39, 39–48. [Google Scholar] [CrossRef]
- Ford, M.; Fleming, R. Mechanical solid-liquid separation of livestock manure. Literature review. In Report to Ontario Pork, Case Study; University of Guelph: Ridgetown, ON, Canada, 2002. [Google Scholar]
- Massé, D.; Croteau, F.; Masse, L. The fate of crop nutrients during digestion of swine manure in psychrophilic anaerobic sequencing batch reactors. Bioresour. Technol. 2007, 98, 2819–2823. [Google Scholar] [CrossRef]
- Tu, Q. Fats, Oils and Greases to Biodiesel: Technology Development and Sustainability Assessment; University of Cincinnati: Cincinnati, OH, USA, 2015. [Google Scholar]
- Tu, Q.; McDonnell, B.E. Monte Carlo analysis of life cycle energy consumption and greenhouse gas (GHG) emission for biodiesel production from trap grease. J. Clean. Prod. 2016, 112, 2674–2683. [Google Scholar] [CrossRef]
- Wallace, T.; Gibbons, D.; O’Dwyer, M.; Curran, T.P. International evolution of fat, oil and grease (FOG) waste management–A review. J. Environ. Manag. 2017, 187, 424–435. [Google Scholar] [CrossRef] [Green Version]

| Common Primary Wastewater Substrates | C/N Ratio | Ref. | |
|---|---|---|---|
| Sewage sludge (SS) | 6–10 | [10] | |
| Waste activated sludge (WAS) | 10 | [11] | |
| Thickened waste activated sludge (TWAS) | 6–9 | [12] | |
| Common Co-Substrates | Example | C/N Ratio | Ref. |
| Organic fraction of municipal solid wastes (OFMSW) | 11–21 | [13] | |
| Food waste (FW) | 11–15 | [14] | |
| Agricultural wastes (AW): | Rice straw | 50–53 | [12] |
| Potatoes | 35–60 | [12] | |
| Corn stalks/straw | 50–56 | [12] | |
| Sugar cane/bagasse | 140–150 | [12] | |
| Sugar beet | 35–40 | [12] | |
| Grass/trimmings | 12–16 | [12] | |
| Fallen leaves | 50–53 | [12] | |
| Horse manure | 20–25 | [12] | |
| Pig manure | 6–14 | [12] | |
| Cow dung | 16–25 | [12] | |
| Crude glycerol (CG) | 68 | [15] | |
| Fat, oil and grease (FOG) | 22 | [7] |
| Primary Substrate | Co-Substrate 1 | Mixing Ratio 2 | Mode 3 (Volume) | T 4 | HRT (d) | OLR (gVS L−1d−1) | VS Removal (%) | Methane Yield (L CH4 g−1 VSadded) | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mono | Co | Improved % | Mono | Co | Improved (%) | |||||||||
| 1 | Sewage sludge | FW | 60:40 VS | C (4 L) | M T-M | 8 7 | 3.5 6.1 | - - | 42 45 | - - | - - | 0.18 0.20 | - - | [29] |
| 2 | Dewatered sludge | FW | 2.4:1 VS 0.9:1 VS 0.4:1 VS | C (6 L) | M | 30 | 4–6 | 38 | 51 62 70 | 34 63 84 | 0.24 | 0.30 0.35 0.40 | 25 46 67 | [14] |
| 3 | Sewage sludge | FVW + FW | 1:0.23 VS 1:2.09 VS | B (1.2 L) | M | 17 26 | - | 50 | 30 40 | −40 −25 | 0.25 | 0.29 0.37 | 18 47 | [19] |
| 4 | Sewage sludge | FVW | 100:20 V | C (100 L) | M | 10–14 | 2.1 | 22 | 24 | 9 | - | 0.38 | - | [30] |
| 5 | Waste activated sludge | OFMSW | 50:50 V | C (380 L) | M T | 23.5 22.3 | 1.6 1.66 | - - | - - | 0.09 | 0.17 0.30 | 89 233 | [24] | |
| 6 | Sewage sludge | OFMSW | 46:54 VS | C (5.5 L) | M | 20 | 1.9 | 36 | 70 | 94 | 0.22 | 0.35 | 59 | [28] |
| 7 | Waste activated sludge | OFMSW | 70:30 W 50:50 W | B (0.2 L) | M | 100 | - | 65 | 58 58 | −11 −11 | 0.25 | 0.22 0.14 | −12 −44 | [25] |
| 8 | Waste untreated sludge | OFMSW | 70:30 W 50:50 W | B (0.2 L) | M | 100 | - | 72 | 66 60 | −8 −17 | 0.44 | 0.29 0.28 | −34 −36 | [25] |
| Primary Substrate | Co- Substrate | Mixing Ratio 1 | Mode 2 (Volume) | T 3 | HRT (d) | OLR (g VS L−1d−1) | VS Removal (%) | Methane Yield (L CH4 g−1 VSadded) | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mono | Co | Improved % | Mono | Co | Improved (%) | |||||||||
| 1 | Sewage sludge | Crude glycerol | 99:1 V | C (3 L) | M | 23–25 | - | −79 | −32 | 60 | 0.26 4 | 0.56 4 | 115 | [38] |
| 2 | Sewage sludge | Crude glycerol | 98.9:1.1 V | SC (1200 L) | M | 20 | 1.04 | 42 | 52 | 24 | 0.62 | 0.87 | 40 | [41] |
| 3 | Pre-treated sewage sludge | Crude glycerol | 99:1 V | SC (9 L) | M | 13 11 9 9 | 1.94 2.11 2.26 1.00 | - | 88–92 | - | 0.76 | 0.92 0.88 0.98 2.10 | 21 16 29 176 | [39] |
| 4 | Primary sludge | Crude glycerol | 124:2−6 W | C (4 L) | M | 32 | 1.25–1.9 | - | - | - | 0.35 | 0.55–0.75 | 57–114 | [42] |
| 5 | Sewage sludge | Crude glycerol | 99.5:0.5 W 98:2 W | C (0.85 L) | M | 17 | 1.81–3.68 | 53 55 | 62 75 | 17 36 | 0.40 0.38 | 0.36 0.34 | −10 −11 | [43] |
| 6 | Sewage sludge | Crude glycerol | 98.8:1.2 V | C (5.5 L) | M | 20 | 1.2 | 36 | 57 | 58 | 0.22 | 0.29 | 31 | [23] |
| 7 | Sewage sludge | Crude glycerol | 99:1 V 99.7:0.3 V 99.5:0.5 V 99.3:0.7 V | B (0.05 L) | M | 15-30 | - | 32.5 | 8 21 19 16 | −75 −35 −42 −51 | 0.045 | 0.010 0.062 0.078 0.064 | −78 38 73 42 | [40] |
| Primary Substrate | Co-Substrate | Mixing Ratio 1 | Mode 2 (Volume) | T 3 | HRT (d) | OLR (g VS L−1 d−1) | VS Removal (%) | Methane Yield (L CH4 g−1 VSadded) | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mono | Co | Improved % | Mono | Co | Improved % | |||||||||
| 1 | Sewage sludge | Sugar beet pulp | - | SC (5 L) | M | 30 20 15 10 6 | 1.1 1.2 1.8 2.1 5 | - | - | - | - | 0.17 0.10 0.16 0.34 0.06 | - | [53] |
| 2 | Waste activated sludge | Olive pomace | 50:50 W | B (1.6 L) | M | 30 | - | 40 | 45 | 12.5 | 0.16 | 0.21 | 31 | [54] |
| 3 | Sewage sludge | Cheese whey | 95:5 V | B (180 L) | M | 24 | 1.1 | 19 | 25 | 32 | 0.25 | 0.36 | 44 | [49] |
| 4 | Primary sludge | Paper pulp reject | 50:50 V | B (0.75 L) | M | 13 | - | - | - | - | 0.16 | 0.37 | 131 | [48] |
| 5 | Waste activated sludge | Olive and grape pomaces | - | B (1.6 L) | M | 30 | - | 35 | 52–55 | 49–57 | 0.06 | 0.07 | 17 | [51] |
| 6 | Sewage sludge | Grape residue Sheep manure Cheese whey | 95:5 V 95:5 V 90:10 V | C (3 L) C (3 L) C (1 L) | M | 24 | 1 1.3 0.8 | 46 38 43 | 41 25 27 | −11 −34 −37 | 0.17 0.17 0.14 | 0.22 0.14 0.31 | 29 −21 121 | [50] |
| 7 | Sewage sludge | Acid cheese whey + brewery spent grain | 90:10 V * 91:9 V * 89:11 V * | SC (40 L) | M | 20 18 16.7 | 1.75 2.9 2.35 | 34 34 46 | 40 42 46 | 18 24 0 | 0.27 0.27 0.18 | 0.26 0.27 0.28 | −4 0 56 | [52] |
| Primary Substrate | Co- Substrate 1 | Mixing Ratio 2 | Mode 3 (Volume) | T 4 | HRT (d) | OLR (g VS L−1 d−1) | VS Removal (%) | Methane Production (L CH4 g−1 VSadded) | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mono | Co | Improved % | Mono | Co | Improved % | |||||||||
| 1 | Sewage sludge | GTS | 90:10 VS 75:25 VS 40:60 VS 90:10 VS 70:30 VS | B (2 L) B (2 L) B (2 L) C (35 L) C (35 L) | M | 37 37 37 13 13 | - - - 2.5 2.4 | - - - 45 45 | - - - 55 58 | - - - 22 29 | 0.29 0.29 0.29 0.24 0.24 | 0.38 0.42 0.60 0.27 0.30 | 31 45 107 13 25 | [69] |
| 2 | Sewage sludge | FOG | 52:48 VS | C (2 L) | M T | 12 13 | 2.45−4.35 | 25 25 | 45 51 | 79 66 | 0.21 0.25 | 0.64 0.67 | 198 169 | [70] |
| 3 | Sewage sludge | GTS | 95:5 VS 80:20 VS 72:28 VS 62:38 VS 54:46 VS 45:55 VS 29:71 VS | SC (4 L) | M | 16 | 1.95 2.19 2.8 3.13 3.46 4.01 4.41 | - | - | - | 0.278 | 0.37 0.44 0.44 0.45 0.46 0.32 0.32 | 34 59 60 61 67 14 13 | [64] |
| 4 | Sewage sludge | GTS | 96:4 VS 77:23 VS 63:37 VS | SC (5.5 L) | M | 20 | 1.2 1.6 1.7 | 36 | 46 52 56 | 28 44 56 | 0.22 | 0.25 0.33 0.29 | 12 48 33 | [71] |
| 5 | Thickened WAS | FOG | 36:64 VS | SC (2 L) | M | 15 | 2.34 | 40 | 57 | 43 | 0.25 | 0.60 | 137 | [7] |
| 6 | Thickened WAS | GW | 93:7 VS 83:13 VS 85:15 VS 78:22 VS 66:34 VS 48:52 VS 26:74 VS 13:87 VS | C (200 L) C (200 L) C (3.4 L) C (3.4 L) C (3.4 L) C (3.4 L) C (3.4 L) C (3.4 L) | M | 30 24 25 26 25 24 24 25 | 1.8 2 1.7 1.6 1.4 1.2 1 0.8 | 29 | 43 36 42 42 39 44 33 17 | 48 24 45 45 35 52 14 −41 | 0.23 | 0.16 0.29 0.29 0.26 0.35 0.48 0.40 0.14 | −32 26 23 11 51 107 72 −40 | [65] |
| 7 | Sewage sludge | GW | 88:12 COD 73:27 COD 63:37 COD | C (5 L) | T | 20 | 1.2 2.1 1.9 | 50 | 46 45 44 | −8 −10 −12 | 0.22 | 0.25 0.23 0.20 | 15 6.5 −6.5 | [72] |
| 8 | Thickened WAS | FOG | 80:20 VS 60:40 VS 40:60 VS 20:80 VS | B (0.25 L) | T | 60 | - | 71 | 74 74 76 47 | 4 4 7 −33 | 0.316 | 0.43 0.45 0.49 0.10 | 35 43 56 −68 | [73] |
| SS | OFMSW/ FW | CG | AW | FOG | ||
|---|---|---|---|---|---|---|
| 1 | Mixing ratio (%) with respect to SS 1 | 100 | 20−50 V | 0.5−1.2 V | 5−50 V | 5−50 VS |
| 2 | Preferred temperature mode 2 | M | T | M | M | M/T |
| 3 | VS removal % | 22−50 | 24−70 | 52−72 | 25−55 | 36−58 |
| 4 | Methane yield (L CH4 g−1 VSadded) | 0.22−0.25 | 0.17−0.38 | 0.29−0.65 | 0.10−0.37 | 0.16−0.50 |
| Evaluation of the effectiveness of implementing anaerobic SS-co-digestion with the co-substrate in a WWTP: | ||||||
| 5 | Benefits and challenges [6,23,39,49,55] |
|
|
|
| |
| 6 | Rating | ★★ | ★★★★ | ★ | ★★★ | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chow, W.L.; Chong, S.; Lim, J.W.; Chan, Y.J.; Chong, M.F.; Tiong, T.J.; Chin, J.K.; Pan, G.-T. Anaerobic Co-Digestion of Wastewater Sludge: A Review of Potential Co-Substrates and Operating Factors for Improved Methane Yield. Processes 2020, 8, 39. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8010039
Chow WL, Chong S, Lim JW, Chan YJ, Chong MF, Tiong TJ, Chin JK, Pan G-T. Anaerobic Co-Digestion of Wastewater Sludge: A Review of Potential Co-Substrates and Operating Factors for Improved Methane Yield. Processes. 2020; 8(1):39. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8010039
Chicago/Turabian StyleChow, Wei Ling, Siewhui Chong, Jun Wei Lim, Yi Jing Chan, Mei Fong Chong, Timm Joyce Tiong, Jit Kai Chin, and Guan-Ting Pan. 2020. "Anaerobic Co-Digestion of Wastewater Sludge: A Review of Potential Co-Substrates and Operating Factors for Improved Methane Yield" Processes 8, no. 1: 39. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8010039