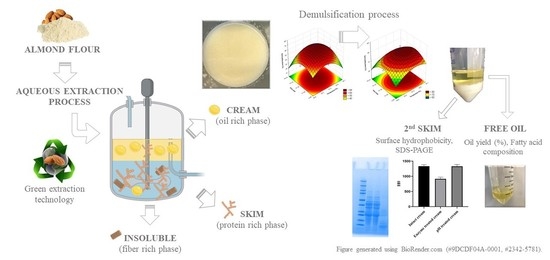
Characterization and Demulsification of the Oil-Rich Emulsion from the Aqueous Extraction Process of Almond Flour
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Processing Scale-Up of the Aqueous Extraction Process (AEP) of Oil and Protein from Almond Flour
2.3. Recovering the Extracted Oil: Enzymatic (ED) and Chemical Demulsification (CD) of the Almond Cream
2.3.1. Enzymatic Demulsification (ED): Tailoring Enzyme Use to Maximize the Cream Demulsification Efficiency
2.3.2. Chemical Demulsification (CD): Effects of Temperature and Incubation Time on the Cream Demulsification Efficiency
2.4. Effects of Demulsification Strategies on the Physicochemical Properties of the Cream Proteins
2.4.1. Low Molecular Weight (MW) Polypeptide Profile Characterization of the Cream Proteins by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.4.2. Surface Hydrophobicity (H0) of the Cream Proteins
2.5. Fatty Acid Composition of the Almond Oil Recovered by Chemical and Enzymatic Demulsification Strategies and Solvent Extraction
2.6. Proximate Analyses
2.7. Statistical Analyses
3. Results and Discussion
3.1. Processing Scale-Up of the Aqueous Extraction Process of Oil and Protein from Almond Flour
3.2. Recovering the Extracted Oil: Enzymatic and Chemical Demulsification of the Cream Emulsion
3.2.1. Enzymatic Demulsification: Tailoring Enzyme Use to Maximize Cream Demulsification Efficiency
3.2.2. Chemical Demulsification: Effects of Temperature and Incubation Time on the Cream Demulsification Efficiency
3.3. Experimental Validation of Optimum Enzymatic and Chemical Demulsification Conditions
3.4. Effects of Demulsification Strategies on the Physicochemical Properties of the Cream Proteins
3.4.1. Low Molecular Weight (MW) Polypeptide Profile Characterization of the Cream Proteins by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
3.4.2. Surface Hydrophobicity (H0) of Cream Proteins
3.5. Fatty Acid Composition of Almond Oil from Chemical and Enzymatic Demulsification Strategies and Solvent Extraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Almond Board of California. Economic Impact of Almond Production; Almond Board of California: Modesto, CA, USA, 2018. [Google Scholar]
- Mandalari, G.; Bisignano, G.; Wickham, M.S.J.; Narbad, A. Potential Prebiotic Properties of Almond (Amygdalus communis L.). Seeds 2008, 74, 4264–4270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Lin, X.; Huang, G.; Zhang, W.; Rao, P.; Ni, L. Prebiotic effects of almonds and almond skins on intestinal microbiota in healthy adult humans. Anaerobe 2014, 26, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Fasoli, E.; D’Amato, A.; Kravchuk, A.; Citterio, A.; Righetti, P.G. In-depth proteomic analysis of non-alcoholic beverages with peptide ligand libraries. I: Almond milk and orgeat syrup. J. Proteom. 2011, 74, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Sathe, S.K. Solubilization, electrophoretic characterization and in vitro digestibility of almond (Prunus amygdalus) proteins. J. Food Biochem. 1993, 16, 249–264. [Google Scholar] [CrossRef]
- Richardson, D.P.; Astrup, A.; Cocaul, A.; Ellis, P. The nutritional and health benefits of almonds: A healthy food choice. Food Sci. Tech. Bull. Funct. Foods 2009, 6, 41–50. [Google Scholar] [CrossRef]
- Ahmad, Z. The uses and properties of almond oil. Complement. Ther. Clin. Pract. 2010, 16, 10–12. [Google Scholar] [CrossRef]
- Savoire, R.; Lanoisellé, J.-L.; Vorobiev, E. Mechanical Continuous Oil Expression from Oilseeds: A Review. Food Bioprocess Technol. 2013, 6, 1–16. [Google Scholar] [CrossRef]
- Johnson, L.A. Recovery of Fats and Oils from Plant and Animal Sources. In Introduction to Fats and Oils; Wan, P.J., Farr, W., Eds.; AOCS Press: Champaign, IL, USA, 2000; pp. 108–135. [Google Scholar]
- Wu, J.; Johnson, L.A.; Jung, S. Demulsification of oil-rich emulsion from enzyme-assisted aqueous extraction of extruded soybean flakes. Bioresour. Technol. 2009, 100, 527–533. [Google Scholar] [CrossRef]
- Environmental Protection Agency. 40 CFR Part 63 National Emissions Standards for Hazardous Air Pollutants: Solvent Extraction for Vegetable Oil Production, Final Rule, Federal Register; Environmental Protection Agency: Washington, DC, USA, 2001; pp. 19005–19026.
- de Moura, J.M.L.N.; Maurer, D.; Jung, S.; Johnson, L.A. Integrated Countercurrent Two-Stage Extraction and Cream Demulsification in Enzyme-Assisted Aqueous Extraction of Soybeans. J. Am. Oil Chem. Soc. 2011, 88, 1045–1051. [Google Scholar] [CrossRef]
- Jung, S.; Moura, J.M.L.N.; Campbell, K.C.; Johnson, L.A. Enzyme-assisted aqueous extraction of oilseeds. In Enhancing Extraction Processes in the Food Industry; Lebovka, N., Vorobiev, E., Chemat, F., Eds.; CRC Press: Boca Raton, FL, USA, 2011; pp. 477–518. [Google Scholar] [CrossRef]
- Ndlela, S.C.; Moura, J.M.L.N.; Olson, N.K.; Johnson, L.A. Aqueous Extraction of Oil and Protein from Soybeans with Subcritical Water. J. Am. Oil Chem. Soc. 2012, 89, 1145–1153. [Google Scholar] [CrossRef]
- Rosenthal, A.; Pyle, D.L.; Niranjan, K. Aqueous and enzymatic processes for edible oil extraction. J. Am. Oil Chem. Soc. 1996, 19, 402–420. [Google Scholar] [CrossRef]
- Campbell, K.A.; Glatz, C.E.; Johnson, L.A.; Jung, S.; Moura, J.M.N.; Kapchie, V.; Murphy, P. Advances in Aqueous Extraction Processing of Soybeans. J. Am. Oil Chem. Soc. 2011, 88, 449–465. [Google Scholar] [CrossRef]
- Balvardi, M.; Rezaei, K.; Mendiola, J.A.; Ibáñez, E. Optimization of the Aqueous Enzymatic Extraction of Oil from Iranian Wild Almond. J. Am. Oil Chem. Soc. 2015, 92, 985–992. [Google Scholar] [CrossRef]
- Yusoff, M.M.; Gordon, M.H.; Niranjan, K. Aqueous enzyme assisted oil extraction from oilseeds and emulsion de-emulsifying methods: A review. Trends Food Sci. Technol. 2015, 41, 60–82. [Google Scholar] [CrossRef]
- Almeida, N.M.; Moura Bell, J.M.L.N.; Johnson, L.A. Properties of Soy Protein Produced by Countercurrent, Two-Stage, Enzyme-Assisted Aqueous Extraction. J. Am. Oil Chem. Soc. 2014, 91, 1077–1085. [Google Scholar] [CrossRef]
- Li, P.; Zhang, W.; Han, X.; Liu, J.; Liu, Y.; Gasmalla, M.A.A.; Yang, R. Demulsification of oil-rich emulsion and characterization of protein hydrolysates from peanut cream emulsion of aqueous extraction processing. J. Food Eng. 2017, 204, 64–72. [Google Scholar] [CrossRef]
- Jiang, L.; Hua, D.; Wang, Z.; Xu, S. Aqueous enzymatic extraction of peanut oil and protein hydrolysates. Food Bioprod. Process. 2010, 88, 233–238. [Google Scholar] [CrossRef]
- Dev, D.K.; Quensel, E. Preparation and Functional Properties of Linseed Protein Products Containing Differing Levels of Mucilage. J. Food Sci. 1988, 53, 1834–1837. [Google Scholar] [CrossRef]
- Tirgar, M.; Silcock, P.; Carne, A.; Birch, E.J. Effect of extraction method on functional properties of flaxseed protein concentrates. Food Chem. 2017, 215, 417–424. [Google Scholar] [CrossRef]
- Aliakbarian, B.; De Faveri, D.; Converti, A.; Perego, P. Optimisation of olive oil extraction by means of enzyme processing aids using response surface methodology. Biochem. Eng. J. 2008, 42, 34–40. [Google Scholar] [CrossRef]
- Najafian, L.; Ghodsvali, A.; Haddad Khodaparast, M.H.; Diosady, L.L. Aqueous extraction of virgin olive oil using industrial enzymes. Food Res. Int. 2009, 42, 171–175. [Google Scholar] [CrossRef]
- Moreau, R.A.; Dickey, L.C.; Johnston, D.B.; Hicks, K.B. A Process for the Aqueous Enzymatic Extraction of Corn Oil from Dry Milled Corn Germ and Enzymatic Wet Milled Corn Germ (E-Germ). J. Am. Oil Chem. Soc. 2009, 86, 469–474. [Google Scholar] [CrossRef]
- Moreau, R.A.; Johnston, D.B.; Powell, M.J.; Hicks, K.B. A comparison of commercial enzymes for the aqueous enzymatic extraction of corn oil from corn germ. J. Am. Oil Chem. Soc. 2004, 81, 1071–1075. [Google Scholar] [CrossRef]
- Almeida, N.M.; Dias, F.F.G.; Rodrigues, M.I.; de Moura Bell, J.M.L.N. Effects of Processing Conditions on the Simultaneous Extraction and Distribution of Oil and Protein from Almond Flour. Processes 2019, 7, 844. [Google Scholar] [CrossRef] [Green Version]
- Souza, T.S.P.; Dias, F.F.G.; Koblitz, M.G.B.; de Moura Bell, J.M.L.N. Effects of enzymatic extraction of oil and protein from almond cake on the physicochemical and functional properties of protein extracts. Food Bioprod. Process. 2020, 122, 280–290. [Google Scholar] [CrossRef]
- Souza, T.S.P.; Dias, F.F.G.; Koblitz, M.G.B.; de Moura Bell, J.M.L.N. Aqueous and Enzymatic Extraction of Oil and Protein from Almond Cake: A Comparative Study. Processes 2019, 7, 472. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Gasmalla, M.A.A.; Liu, J.; Zhang, W.; Yang, R.; Aboagarib, E.A.A. Characterization and demusification of cream emulsion from aqueous extraction of peanut. J. Food Eng. 2016, 185, 62–71. [Google Scholar] [CrossRef]
- Moura, J.M.L.N.; Almeida, N.M.; Johnson, L.A. Scale-up of Enzyme-Assisted Aqueous Extraction Processing of Soybeans. J. Am. Oil Chem. Soc. 2009, 86, 809–815. [Google Scholar] [CrossRef]
- Chabrand, R.M.; Kim, H.J.; Zhang, C.; Glatz, C.E.; Jung, S. Destabilization of the emulsion formed during aqueous extraction of Soybean oil. J. Am. Oil Chem. Soc. 2008, 85, 383–390. [Google Scholar] [CrossRef]
- Damodaran, S.; Anand, K. Sulfhydryl−Disulfide Interchange-Induced Interparticle Protein Polymerization in Whey Protein-Stabilized Emulsions and Its Relation to Emulsion Stability. J. Agric. Food Chem. 1997, 45, 3813–3820. [Google Scholar] [CrossRef]
- Chabrand, R.M.; Glatz, C.E. Destabilization of the emulsion formed during the enzyme-assisted aqueous extraction of oil from soybean flour. Enzym. Microb. Technol. 2009, 45, 28–35. [Google Scholar] [CrossRef]
- Moura, J.M.L.N.; Campbell, K.; Mahfuz, A.; Jung, S.; Glatz, C.E.; Johnson, L. Enzyme-Assisted Aqueous Extraction of Oil and Protein from Soybeans and Cream De-emulsification. J. Am. Oil Chem. Soc. 2008, 85, 985–995. [Google Scholar] [CrossRef]
- Tabtabaei, S.; Diosady, L.L. Aqueous and enzymatic extraction processes for the production of food-grade proteins and industrial oil from dehulled yellow mustard flour. Food Res. Int. 2013, 52, 547–556. [Google Scholar] [CrossRef]
- de Aquino, D.S.; Fanhani, A.; Stevanato, N.; da Silva, C. Sunflower oil from enzymatic aqueous extraction process: Maximization of free oil yield and oil characterization. J. Food Process Eng. 2019, 42, e13169. [Google Scholar] [CrossRef]
- Sathe, S.K.; Wolf, W.J.; Roux, K.H.; Teuber, S.S.; Venkatachalam, M.; Sze-Tao, K.W.C. Biochemical Characterization of Amandin, the Major Storage Protein in Almond (Prunus dulcis L.). J. Agric. Food Chem. 2002, 50, 4333–4341. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, W.; Yang, R.; Ahmed, M.A.; Hua, X.; Zhang, W.; Zhang, Y. Preparation and functional properties of protein from heat-denatured soybean meal assisted by steam flash-explosion with dilute acid soaking. J. Food Process Eng. 2013, 119, 56–64. [Google Scholar] [CrossRef] [Green Version]
- American Oil Chemists’ Society AOCS Standard Procedure Am 5-04. Rapid Determination of Oil/Fat Utilizing High-Temperature Solvent Extraction. In Official Methods and Recommended Practices of AOCS; American Oil Chemists’ Society: Urbana, IL, USA, 2017. [Google Scholar]
- Association of Official Analytical Chemists. Official Method 922.06 Fat in Flour. Acid Hydrolysis Method. In Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Rockville, MD, USA, 2012. [Google Scholar]
- AACC Approved Methods of Analysis, 11th Edition—AACC Method 44-40.01, Moisture—Modified Vacuum-Oven Method. Available online: http://methods.aaccnet.org/summaries/44-40-01.aspx (accessed on 6 August 2020).
- Jung, S. Aqueous extraction of oil and protein from soybean and lupin: A comparative study. Food Process. Preserv. 2009, 33, 547–559. [Google Scholar] [CrossRef]
- Zhang, S.B.; Lu, Q.Y. Characterizing the structural and surface properties of proteins isolated before and after enzymatic demulsification of the aqueous extract emulsion of peanut seeds. Food Hydrocoll. 2015, 47, 51–60. [Google Scholar] [CrossRef]
- Kinsella, J.E. Functional properties of soy proteins. J. Am. Oil Chem. Soc. 1979, 56, 242–258. [Google Scholar] [CrossRef]
- Huang, A.H.C. Oil bodies and oleosins in seeds. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 177–200. [Google Scholar] [CrossRef]
- Beisson, F.; Ferté, N.; Voultoury, R.; Arondel, V. Large scale purification of an almond oleosin using an organic solvent procedure. Plant Physiol. Biochem. 2001, 39, 623–630. [Google Scholar] [CrossRef]
- Ghribi, A.M.; Gafsi, I.M.; Sila, A.; Blecker, C.; Danthine, S.; Attia, H.; Bougatef, A.; Besbes, S. Effects of enzymatic hydrolysis on conformational and functional properties of chickpea protein isolate. Food Chem. 2015, 187, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Surówka, K.; Zmudziński, D.; Surówka, J. Enzymatic modification of extruded soy protein concentrates as a method of obtaining new functional food components. Trends Food Sci. Technol. 2004, 15, 153–160. [Google Scholar] [CrossRef]
- Jung, S.; Murphy, P.A.; Johnson, L.A. Physicochemical and Functional Properties of Soy Protein Substrates Modified by Low Levels of Protease Hydrolysis. J. Food Sci. 2005, 70, C180–C187. [Google Scholar] [CrossRef]
- Avramenko, N.A.; Low, N.H.; Nickerson, M.T. The effects of limited enzymatic hydrolysis on the physicochemical and emulsifying properties of a lentil protein isolate. Food Res. Int. 2013, 51, 162–169. [Google Scholar] [CrossRef]
- Sathe, S.K.; Seeram, N.P.; Kshirsagar, H.H.; Heber, D.; Lapsley, K.A. Fatty acid composition of California grown almonds. J. Food Sci. 2008, 73. [Google Scholar] [CrossRef]
- Latif, S.; Anwar, F. Aqueous enzymatic sesame oil and protein extraction. Food Chem. 2011, 125, 679–684. [Google Scholar] [CrossRef]




| Experiments | Experimental Parameters | Demulsification Yield (%) | |||
|---|---|---|---|---|---|
| X1 (pH) | X2 (T, °C) | X3 (t, min) | X4 (E, %) | ||
| 1 | −1 (6.0) | −1 (50.0) | −1 (30) | −1 (0.5) | 18.6 |
| 2 | 1 (9.0) | −1 (50.0) | −1 (30) | 1 (2.5) | 20.4 |
| 3 | −1 (6.0) | 1 (65.0) | −1 (30) | 1 (2.5) | 17.8 |
| 4 | 1 (9.0) | 1 (65.0) | −1 (30) | −1 (0.5) | 49.6 |
| 5 | −1 (6.0) | −1 (50.0) | 1 (90) | 1 (2.5) | 19.2 |
| 6 | 1 (9.0) | −1 (50.0) | 1 (90) | −1 (0.5) | 55.6 |
| 7 | −1 (6.0) | 1 (65.0) | 1 (90) | −1 (0.5) | 24.3 |
| 8 | 1 (9.0) | 1 (65.0) | 1 (90) | 1 (2.5) | 31.2 |
| 9 | 0 (7.5) | 0 (57.5) | 0 (60) | 0 (1.5) | 47.4 |
| 10 | 0 (7.5) | 0 (57.5) | 0 (60) | 0 (1.5) | 49.8 |
| 11 | 0 (7.5) | 0 (57.5) | 0 (60) | 0 (1.5) | 48.4 |
| Variables * | Effect | p-Value |
|---|---|---|
| pH (X1) | 19.96 | 0.02 |
| Temperature (°C) (X2) | 1.52 | 0.80 |
| Time (min) (X3) | 6.75 | 0.30 |
| Enzyme (%) (w/w) (X4) | −15.62 | 0.05 |
| Experiments | Experimental Parameters | Demulsification Yield (%) | |
|---|---|---|---|
| X1 (pH) | X2 (E, %) | ||
| 1 | −1 (7.0) | −1 (0.3) | 50.0 |
| 2 | 1 (9.0) | −1 (0.3) | 40.4 |
| 3 | −1 (7.0) | 1 (1.3) | 39.0 |
| 4 | 1 (9.0) | 1 (1.3) | 42.6 |
| 5 | 0 (8.0) | 0 (0.8) | 60.2 |
| 6 | 0 (8.0) | 0 (0.8) | 62.1 |
| 7 | 0 (8.0) | 0 (0.8) | 67.1 |
| 8 | −α * (6.6) | 0 (0.8) | 37.2 |
| 9 | α (9.4) | 0 (0.8) | 46.2 |
| 10 | 0 (8.0) | −α (0.1) | 42.9 |
| 11 | 0 (8.0) | α (1.5) | 39.5 |
| Response | Equation | F-Test | R2 | p-Value |
|---|---|---|---|---|
| ED | 5.64 | 0.8639 | 0.00034 | |
| CD | 5.14 | 0.9368 | 0.00096 |
| Experiments | Experimental Parameters | Demulsification Yield (%) | |
|---|---|---|---|
| X1 (T, °C) | X2 (t, min) | ||
| 1 | −1 (50.0) | −1 (15.0) | 49.9 |
| 2 | 1 (65.5) | −1 (15.0) | 47.7 |
| 3 | −1 (50.0) | 1 (60.0) | 59.0 |
| 4 | 1 (65.5) | 1 (60.0) | 46.2 |
| 5 | 0 (57.5) | 0 (37.5) | 67.4 |
| 6 | 0 (57.5) | 0 (37.5) | 69.3 |
| 7 | 0 (57.5) | 0 (37.5) | 65.3 |
| 8 | −α* (46.96) | 0 (37.5) | 53.7 |
| 9 | α (68.1) | 0 (37.5) | 43.9 |
| 10 | 0 (57.5) | −α (5.8) | 42.5 |
| 11 | 0 (57.5) | α (69.2) | 57.9 |
| Treatment | Experimental Conditions | Demulsification Yield (%) | |
|---|---|---|---|
| Predicted * | Experimental Validation ** | ||
| Chemical | pH 5.0, 55 °C, 46 min, no enzyme | 68.14 a | 65.53 ± 2.29 a,A |
| Enzymatic | pH 8.0, 50 °C, 30 min, 0.8% (w/w) of enzyme | 63.11 a | 65.77 ± 2.77 a,A |
| Control | pH 9.0, 50 °C, 60 min, no enzyme | - | 7.85 ± 0.51 B |
| Fatty Acid (%, w/w *) | Enzymatic Demulsification | Chemical Demulsification | Solvent Extraction |
|---|---|---|---|
| C16:0 | 5.01 ± 0.4 | 5.50 ± 0.54 | 4.58 ± 0.36 |
| C16:1 | 0.24 ± 0.02 | 0.28 ± 0.05 | 0.20 ± 0.04 |
| C18:0 | 0.42 ± 0.07 | 0.46 ± 0.06 | 0.45 ± 0.04 |
| C18:1 (cis) | 71.73 ± 1.03 | 72.85 ± 1.38 | 74.37 ± 2.12 |
| C18:2 (n-6) | 22.55 ± 0.60 | 20.86 ± 1.80 | 20.33 ± 1.75 |
| C18:3 (n-3) | 0.03 ± 0.001 | 0.02 ± 0.001 | 0.05 ± 0.002 |
| C20:0 | 0.03 ± 0.002 | 0.03 ± 0.001 | 0.02 ± 0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
F. G. Dias, F.; de Almeida, N.M.; S. P. de Souza, T.; Taha, A.Y.; L. N. de Moura Bell, J.M. Characterization and Demulsification of the Oil-Rich Emulsion from the Aqueous Extraction Process of Almond Flour. Processes 2020, 8, 1228. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8101228
F. G. Dias F, de Almeida NM, S. P. de Souza T, Taha AY, L. N. de Moura Bell JM. Characterization and Demulsification of the Oil-Rich Emulsion from the Aqueous Extraction Process of Almond Flour. Processes. 2020; 8(10):1228. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8101228
Chicago/Turabian StyleF. G. Dias, Fernanda, Neiva M. de Almeida, Thaiza S. P. de Souza, Ameer Y. Taha, and Juliana M. L. N. de Moura Bell. 2020. "Characterization and Demulsification of the Oil-Rich Emulsion from the Aqueous Extraction Process of Almond Flour" Processes 8, no. 10: 1228. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8101228