Computer-Aided Nonlinear Frequency Response Method for Investigating the Dynamics of Chemical Engineering Systems
Abstract
:1. Introduction
- 1.
- Developing experimental techniques for investigating process equilibrium and kinetics and estimating the related parameters.
- 2.
- Developing a computational method for direct prediction of the periodic steady-state of cyclic processes.
- 3.
- Developing a method for fast and easy evaluation of possible enhancement of process performances through forced periodic modulations of the system inputs.
2. Software Application for Computer-Aided Nonlinear Frequency Response Method
3. Examples of the Application of the cNFR Method
3.1. Example 1: Analysis of Forced Periodic Operation of Isothermal Continuous Stirred-Tank Reactor with a Simple Reaction Mechanism (CSTR)
3.1.1. Problem Formulation
3.1.2. Filling in the cNFR Interface and Generating Results
3.1.3. Simulating the FRFs Derived Using cNFR and its Verification
- For modulation of the inlet reaction concentration, (input ):
- For modulation of the volumetric flow rate, (input ):
- The cross-function:
3.1.4. Analyzing System Stability
3.1.5. Analyzing System Forced Periodic Operation
3.2. Example 2: Analysis of an Electrochemical Reaction Process (ECR)—Comparison with Experimental Results
3.2.1. Problem Formulation
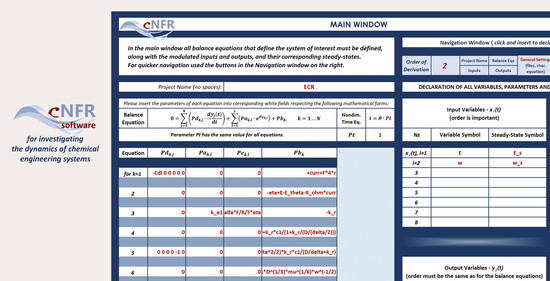
3.2.2. Filling in the cNFR Interface and Generating Results
3.2.3. Comparison with Experimental FRFs and Model Identification
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kramers, H.; Alberda, G. Frequency response analysis of continuous flow systems. Chem. Eng. Sci. 1953, 2, 173–181. [Google Scholar] [CrossRef]
- Deisler, P.F.; Wilhelm, R.H. Diffusion in beds of porous solids. Measurement by frequency response techniques. Ind. Eng. Chem. 1953, 45, 1219–1227. [Google Scholar]
- Turner, G.A. The frequency response of some illustrative models of porous media: Experiments and computations with two artificial packed beds to illustrate a method of determining parameters of the bed. Chem. Eng. Sci. 1959, 10, 14–21. [Google Scholar] [CrossRef]
- Ritter, A.B.; Douglas, J.M. Frequency response of nonlinear systems. Ind. Eng. Chem. Fundam. 1970, 9, 21–28. [Google Scholar] [CrossRef]
- Gray, R.I.; Prados, J.W. The dynamics of a packed gas absorber by frequency response analysis. AIChE J. 1963, 9, 211–216. [Google Scholar] [CrossRef]
- Devereux, B.M.; Denn, M.M. Frequency response analysis of polymer melt spinning. Ind. Eng. Chem. Res. 1994, 33, 2384–2390. [Google Scholar] [CrossRef]
- Mehta, S.C.; Shemilt, L.W. Frequency response of liquid fluidized systems. Part ii effect of liquid viscosity. Can. J. Chem. Eng. 1976, 54, 43–51. [Google Scholar]
- Stephanopoulos, G. Chemical Process Control—An Introduction to Theory and Practice; Prentice Hall: Inglewood Cliffs, NJ, USA, 1980. [Google Scholar]
- Weiner, D.D.; Spina, J.F. Sinusoidal Analysis and Modeling of Weakly Nonlinear Circuits; Van Nostrand: New York, NY, USA, 1980. [Google Scholar]
- Volterra, V. Theory of Functionals and of Integral and Integro-Differential Equations; Dover: New York, NY, USA, 1959. [Google Scholar]
- Bracewell, R.N. The Fourier Transform and Its Applications; McGraw-Hill: New York, NY, USA, 1986. [Google Scholar]
- Chatterjee, A.; Vyas, N.S. Convergence analysis of volterra series response of nonlinear systems subjected to harmonic excitation. J. Sound Vib. 2000, 236, 339–358. [Google Scholar] [CrossRef] [Green Version]
- Edwards, W.M.; Kim, H.N. Multiple Steady States in FCC Unit Operations. In Tenth International Symposium on Chemical Reaction Engineering; Pergamon: Oxford, UK, 1988; pp. 1825–1830. [Google Scholar]
- Petkovska, M.; Do, D.D. Nonlinear frequency response of adsorption systems: Isothermal batch and continuous flow adsorbers. Chem. Eng. Sci. 1998, 53, 3081–3097. [Google Scholar] [CrossRef]
- Petkovska, M.; Do, D.D. Use of higher-order frequency response functions for identification of nonlinear adsorption kinetics: Single mechanisms under isothermal conditions. Nonlinear Dyn. 2000, 21, 353–376. [Google Scholar] [CrossRef]
- Petkovska, M.; Petkovska, L.T. Use of nonlinear frequency response for discriminating adsorption kinetics mechanisms resulting with bimodal characteristic functions. Adsorption 2003, 9, 133–142. [Google Scholar] [CrossRef]
- Petkovska, M.; Seidel-Morgenstern, A. Nonlinear frequency response of a chromatographic column. Part i: Application to estimation of adsorption isotherms with inflection points. Chem. Eng. Commun. 2005, 192, 1300–1333. [Google Scholar]
- Petkovska, M.; Marković, A. Fast estimation of quasi-steady states of cyclic nonlinear processes based on higher-order frequency response functions. Case study: Cyclic operation of an adsorption column. Ind. Eng. Chem. Res. 2006, 45, 266–291. [Google Scholar]
- Petkovska, M. Nonlinear fr-zlc method for investigation of adsorption equilibrium and kinetics. Adsorption 2008, 14, 223–239. [Google Scholar] [CrossRef]
- Marković, A.; Seidel-Morgenstern, A.; Petkovska, M. Evaluation of the potential of periodically operated reactors based on the second order frequency response function. Chem. Eng. Res. Des. 2008, 86, 682–691. [Google Scholar] [CrossRef]
- Bensmann, B.; Petkovska, M.; Vidaković-Koch, T.; Hanke-Rauschenbach, R.; Sundmacher, K. Nonlinear frequency response of electrochemical methanol oxidation kinetics: A theoretical analysis. J. Electrochem. Soc. 2010, 157, B1279–B1289. [Google Scholar] [CrossRef]
- Vidaković-Koch, T.R.; Panić, V.V.; Andrić, M.; Petkovska, M.; Sundmacher, K. Nonlinear frequency response analysis of the ferrocyanide oxidation kinetics. Part i. A theoretical analysis. J. Phys. Chem. C 2011, 115, 17341–17351. [Google Scholar]
- Panić, V.V.; Vidaković-Koch, T.R.; Andrić, M.; Petkovska, M.; Sundmacher, K. Nonlinear frequency response analysis of the ferrocyanide oxidation kinetics. Part ii. Measurement routine and experimental validation. J. Phys. Chem. C 2011, 115, 17352–17358. [Google Scholar]
- Brzić, D.; Petkovska, M. A study of applicability of nonlinear frequency response method for investigation of gas adsorption based on numerical experiments. Ind. Eng. Chem. Res. 2013, 52, 16341–16351. [Google Scholar] [CrossRef]
- Nikolic Paunic, D.; Petkovska, M. Evaluation of periodic processes with two modulated inputs based on nonlinear frequency response analysis. Case study: Cstr with modulation of the inlet concentration and flow-rate. Chem. Eng. Sci. 2013, 104, 208–219. [Google Scholar]
- Nikolić, D.; Seidel-Morgenstern, A.; Petkovska, M. Nonlinear frequency response analysis of forced periodic operation of non-isothermal cstr using single input modulations. Part i: Modulation of inlet concentration or flow-rate. Chem. Eng. Sci. 2014, 117, 71–84. [Google Scholar]
- Nikolić, D.; Seidel-Morgenstern, A.; Petkovska, M. Nonlinear frequency response analysis of forced periodic operation of non-isothermal cstr using single input modulations. Part ii: Modulation of inlet temperature or temperature of the cooling/heating fluid. Chem. Eng. Sci. 2014, 117, 31–44. [Google Scholar]
- Brzić, D.; Petkovska, M. Nonlinear frequency response analysis of nonisothermal adsorption controlled by macropore diffusion. Chem. Eng. Sci. 2014, 118, 141–153. [Google Scholar] [CrossRef]
- Brzić, D.; Petkovska, M. Nonlinear frequency response measurements of gas adsorption equilibrium and kinetics: New apparatus and experimental verification. Chem. Eng. Sci. 2015, 132, 9–21. [Google Scholar] [CrossRef]
- Petkovska, M. Nonlinear frequency response of nonisothermal adsorption controlled by micropore diffusion with variable diffusivity. J. Serb. Chem. Soc. 2000, 65, 939–961. [Google Scholar] [CrossRef]
- Petkovska, M. Nonlinear frequency response of nonisothermal adsorption systems. Nonlinear Dyn. 2001, 26, 351–370. [Google Scholar] [CrossRef]
- Ilić, M.; Petkovska, M.; Seidel-Morgenstern, A. Nonlinear frequency response functions of a chromatographic column—A critical evaluation of their potential for estimation of single solute adsorption isotherms. Chem. Eng. Sci. 2007, 62, 1269–1281. [Google Scholar] [CrossRef]
- Ilić, M.; Petkovska, M.; Seidel-Morgenstern, A. Nonlinear frequency response method for estimation of single solute adsorption isotherms. Part i. Theoretical basis and simulations. Chem. Eng. Sci. 2007, 62, 4379–4393. [Google Scholar] [CrossRef]
- Ilić, M.; Petkovska, M.; Seidel-Morgenstern, A. Nonlinear frequency response method for estimation of single solute adsorption isotherms. Part ii. Experimental study. Chem. Eng. Sci. 2007, 62, 4394–4408. [Google Scholar] [CrossRef]
- Ilić, M.; Petkovska, M.; Seidel-Morgenstern, A. Theoretical investigation of the adsorption of a binary mixture in a chromatographic column using the nonlinear frequency response technique. Adsorption 2007, 13, 541–567. [Google Scholar] [CrossRef]
- Ilić, M.; Petkovska, M.; Seidel-Morgenstern, A. Determination of competitive adsorption isotherms applying the nonlinear frequency response method. Part i. Theoretical analysis. J. Chromatogr. A 2009, 1216, 6098–6107. [Google Scholar] [CrossRef] [PubMed]
- Ilić, M.; Petkovska, M.; Seidel-Morgenstern, A. Determination of competitive adsorption isotherms applying the nonlinear frequency response method: Part ii. Experimental demonstration. J. Chromatogr. A 2009, 1216, 6108–6118. [Google Scholar] [CrossRef] [PubMed]
- Brzić, D.; Petkovska, M. Some practical aspects of nonlinear frequency response method for investigation of adsorption equilibrium and kinetics. Chem. Eng. Sci. 2012, 82, 62–72. [Google Scholar] [CrossRef]
- Kandaswamy, S.; Sorrentino, A.; Borate, S.; Živković, L.A.; Petkovska, M.; Vidaković-Koch, T. Oxygen reduction reaction on silver electrodes under strong alkaline conditions. Electrochim. Acta 2019, 320, 134517. [Google Scholar] [CrossRef]
- Naphtali, L.M.; Polinski, L.M. A novel technique for characterization of adsorption rates on heterogeneous surfaces. J. Phys. Chem. 1963, 67, 369–375. [Google Scholar] [CrossRef]
- Epelboin, I.; Loric, G. Sur un phénomène de résonance observé en basse fréquence au cours des électrolyses accompagnées d’une forte surtension anodique. J. Phys. Radium 1960, 21, 74–76. [Google Scholar] [CrossRef] [Green Version]
- Petkovska, M. Application of nonlinear frequency response to adsorption systems with complex kinetic mechanisms. Adsorption 2005, 11, 497–502. [Google Scholar] [CrossRef]
- Petkovska, M. Nonlinear Frequency Response Method for Investigation of Equilibria and Kinetics of Adsorption Systems. In Finely Dispersed Particles: Micro-, Nano-, and Atto-Engineering; Spasic, A.M., Hsu, J.-P., Eds.; CRC Taylor & Francis: Boca Raton, FL, USA, 2005; pp. 283–327. [Google Scholar]
- Petkovska, M.; Seidel-Morgenstern, A. Evaluation of Periodic Processes. In Periodic Operation of Reactors; Silveston, P.L., Hudgins, R.R., Eds.; Butterworth-Heinemann Elsevier: Oxford, UK, 2013; pp. 387–413. [Google Scholar]
- Nikolić, D.; Seidel-Morgenstern, A.; Petkovska, M. Nonlinear frequency response analysis of forced periodic operation of non-isothermal cstr with simultaneous modulation of inlet concentration and inlet temperature. Chem. Eng. Sci. 2015, 137, 40–58. [Google Scholar] [CrossRef] [Green Version]
- Nikolić, D.; Seidel-Morgenstern, A.; Petkovska, M. Periodic operation with modulation of inlet concentration and flow rate. Part i: Nonisothermal continuous stirred-tank reactor. Chem. Eng. Technol. 2016, 39, 2020–2028. [Google Scholar]
- Nikolić, D.; Petkovska, M. Evaluation of performance of periodically operated reactors for single input modulations of general waveforms. Chem. Ing. Tech. 2016, 88, 1715–1722. [Google Scholar] [CrossRef]
- Nikolić, D.; Felischak, M.; Seidel-Morgenstern, A.; Petkovska, M. Periodic operation with modulation of inlet concentration and flow rate part ii: Adiabatic continuous stirred-tank reactor. Chem. Eng. Technol. 2016, 39, 2126–2134. [Google Scholar] [CrossRef]
- Nikolic Paunic, D. Forced Periodically Operated Chemical Reactors-Evaluation and Analysis by the Nonlinear Frequency Response Method; Stage of Publication (Under Review); Faculty of Technology and Metalurgy, University of Belgrade: Belgrade, Serbia, 2016. [Google Scholar]
- Currie, R.; Nikolic, D.; Petkovska, M.; Simakov, D.S.A. CO2 conversion enhancement in a periodically operated sabatier reactor: Nonlinear frequency response analysis and simulation-based study. Isr. J. Chem. 2018, 58, 762–775. [Google Scholar] [CrossRef]
- Petkovska, M.; Nikolić, D.; Seidel-Morgenstern, A. Nonlinear frequency response method for evaluating forced periodic operations of chemical reactors. Isr. J. Chem. 2018, 58, 663–681. [Google Scholar] [CrossRef]
- Nikolić, D.; Seidel-Morgenstern, A.; Petkovska, M. Nonlinear frequency response analysis of forced periodic operations with simultaneous modulation of two general waveform inputs with applications on adiabatic cstr with square-wave modulations. Chem. Eng. Sci. 2020, 226, 115842. [Google Scholar] [CrossRef]
- Petkovska, M.; Nikolić, D.; Marković, A.; Seidel-Morgenstern, A. Fast evaluation of periodic operation of a heterogeneous reactor based on nonlinear frequency response analysis. Chem. Eng. Sci. 2010, 65, 3632–3637. [Google Scholar] [CrossRef]















| Label | Units | Value |
|---|---|---|
| kmol/m3 | 16.02 | |
| m3/min | 0.0472 | |
| m3 | 28.32 | |
| m3/kmol/min | 1.248 | |
| / | 2 | |
| / | 0.9 | |
| / | 0.9 |
| Label | Units | Value in 0.1 M NaOH | Value in 11 M NaOH |
|---|---|---|---|
| mol/m3 | 1.18 | 0.05 | |
| F/m2 | 1.15 | 1.40 | |
| m2/s | 1.9 × 10−9 | 6.0 × 10−11 | |
| m/s | 4.7 × 10−9 | 5.5 × 10−10 | |
| V | 1.222 | 1.171 | |
| m2/s | 1.0128 × 10−6 | 1.2800 × 10−5 | |
| Ω m2 | 9.731 × 10−4 | 1.111 × 10−4 | |
| / | 0.445 | 0.500 | |
| V | 0.9 | ||
| rpm | 1600 | ||
| K | 293.15 | ||
| C/mol | 96485 | ||
| J/mol/K | 8.314 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Živković, L.A.; Vidaković-Koch, T.; Petkovska, M. Computer-Aided Nonlinear Frequency Response Method for Investigating the Dynamics of Chemical Engineering Systems. Processes 2020, 8, 1354. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8111354
Živković LA, Vidaković-Koch T, Petkovska M. Computer-Aided Nonlinear Frequency Response Method for Investigating the Dynamics of Chemical Engineering Systems. Processes. 2020; 8(11):1354. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8111354
Chicago/Turabian StyleŽivković, Luka A., Tanja Vidaković-Koch, and Menka Petkovska. 2020. "Computer-Aided Nonlinear Frequency Response Method for Investigating the Dynamics of Chemical Engineering Systems" Processes 8, no. 11: 1354. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8111354