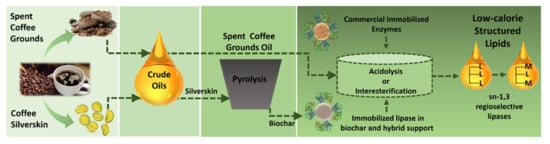
Synthesis of Dietetic Structured Lipids from Spent Coffee Grounds Crude Oil Catalyzed by Commercial Immobilized Lipases and Immobilized Rhizopus oryzae Lipase on Biochar and Hybrid Support
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of the Proximate Composition of Coffee Silverskin and Spent Coffee Grounds
2.3. Oil Extraction from Spent Coffee Grounds
2.4. Quality Parameters and Fatty Acid Composition of Spent Coffee Grounds and Coffee Silverskin Crude Oils
2.5. Preparation of Supports
2.6. ROL Immobilization
2.7. Determination of Hydrolytic Activity
2.8. Acidolysis and Interesterification Reactions
2.9. Batch Operational Stability Tests
2.10. Analysis of Reaction Products
2.11. Statistical Analysis
3. Results and Discussion
3.1. Proximate Composition of Coffee Silverskin and Spent Coffee Grounds and Characterization of Respective Oils
3.2. Determination of Hydrolytic Activity
3.3. Screening of Biocatalysts in Acidolysis and Interesterification Reactions
3.4. Acidolysis and Interesterification Kinetics
3.5. Batch Operational Stability Tests
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Coffee Organization—ICO. Available online: http://www.ico.org/prices/pr-prices.pdf (accessed on 12 July 2020).
- Salazar-López, N.J.; López-Rodríguez, C.V.; Hernández-Montoya, D.A.; Campos-Vega, R. Health Benefits of Spent Coffee Grounds Coffea Arabica L. Generalities. In Food Wastes and By-Products: Nutraceutical and Health Potential; Campos-Vega, R., Dave Oomah, B., Vergara, H., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2020. [Google Scholar]
- Tarigan, J.B.; Ginting, M.; Mubarokah, S.N.; Sebayang, F.; Karo-karo, J.; Nguyen, T.T.; Ginting, J.; Sitepu, E.K. Direct biodiesel production from wet spent coffee grounds. RSC Adv. View 2019, 9, 35109–35116. [Google Scholar] [CrossRef] [Green Version]
- Galanakis, C. Food waste valorization opportunities for different food industries. In The Interaction of Food Industry and Environment; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 341–422. ISBN 9780128164495. [Google Scholar]
- Corrêa, C.L.O.; Penha, E.M.; Freitas-Silva, O.; Luna, A.S.; Gottschalk, L.M.F. Enzymatic Technology Application on Coffee Co-products: A Review. Waste Biomass Valorization 2020. [Google Scholar] [CrossRef]
- Yang, H.; Sombatngamwilai, T.; Yu, W.; Kuo, M. Drying Applications during Value-Added Sustainable Processing for Selected Mass-Produced Food Coproducts. Processes 2020, 8, 307. [Google Scholar] [CrossRef] [Green Version]
- Polidoro, S.; Scapin, E.; Lazzari, E.; Nunes, A. Valorization of co ff ee silverskin industrial waste by pyrolysis: From optimization of bio-oil production to chemical characterization by GC × GC/qMS. J. Anal. Appl. Pyrolysis 2018, 129, 43–52. [Google Scholar] [CrossRef]
- González, M.E.; Cea, M.; Sangaletti, N.; González, A.; Toro, C.; Diez, M.C.; Moreno, N.; Querol, X.; Navia, R. Biochar derived from agricultural and forestry residual biomass: Characterization and potential application for enzymes immobilization. J. Biobased Mater. Bioenergy 2013, 7, 724–732. [Google Scholar] [CrossRef]
- Almeida, L.C.; Barbosa, A.S.; Fricks, A.T.; Freitas, L.S.; Lima, Á.S.; Soares, C.M.F. Use of conventional or non-conventional treatments of biochar for lipase immobilization. Process Biochem. 2017, 61, 124–129. [Google Scholar] [CrossRef]
- Ribeiro, L.M.O.; Meili, L.; Gois, G.N.S.B.; Almeida, R.M.; Duarte, J.L.d.S. Immobilization of lipase in biochar obtained from Manihot esculenta Crantz. Rev. Ion 2019, 32, 7–13. [Google Scholar] [CrossRef]
- Costa, A.S.G.; Alves, R.C.; Vinha, A.F.; Costa, E.; Costa, C.S.G.; Nunes, M.A.; Almeida, A.A.; Santos-silva, A.; Oliveira, M.B.P.P. Nutritional, chemical and antioxidant/pro-oxidant profiles of silverskin, a coffee roasting by-product. Food Chem. 2018, 267, 28–35. [Google Scholar] [CrossRef]
- Ramos-Andrés, M.; Andrés-Iglesias, C.; García-Serna, J. Production of molecular weight fractionated hemicelluloses hydrolyzates from spent coffee grounds combining hydrothermal extraction and a multistep ultra filtration/diafiltration. Bioresour. Technol. 2019, 292, 121940. [Google Scholar] [CrossRef]
- Toschi, T.G.; Cardenia, V.; Bonaga, G.; Mandrioli, M.; Rodriguez-estrada, M.T. Coffee Silverskin: Characterization, Possible Uses, and Safety Aspects. J. Agric. Food Chem. 2014, 62, 10836–10844. [Google Scholar] [CrossRef]
- Ribeiro, H.; Marto, J.; Raposo, S.; Agapito, M.; Isaac, V.; Chiari, B.G.; Lisboa, P.F.; Paiva, A.; Barreiros, S.; Simões, P. From coffee industry waste materials to skin-friendly products with improved skin fat levels. Eur. J. Lipid Sci. Technol. 2013, 115, 330–336. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Nguyen, M.L.; Wang, F.M.; Juan, H.Y.; Su, C.H. Biodiesel production by direct transesterification of wet spent coffee grounds using switchable solvent as a catalyst and solvent. Bioresour. Technol. 2020, 296, 122334. [Google Scholar] [CrossRef] [PubMed]
- Banu, J.R.; Kavitha, S.; Kannah, R.Y.; Kumar, M.D.; Preethi, J.; Atabani, A.E.; Kumar, G. Biorefinery of spent coffee grounds waste: Viable pathway towards circular bioeconomy. Bioresour. Technol. 2020, 302, 122821. [Google Scholar] [CrossRef] [PubMed]
- Passadis, K.; Fragoulis, V.; Stoumpou, V.; Novakovic, J.; Barampouti, E.M.; Mai, S.; Moustakas, K.; Malamis, D.; Loizidou, M. Study of Valorisation Routes of Spent Coffee Grounds. Waste Biomass Valorization 2020, 11, 5295–5306. [Google Scholar] [CrossRef]
- Dang, C.H.; Nguyen, T.D. Physicochemical Characterization of Robusta Spent Coffee Ground Oil for Biodiesel Manufacturing. Waste Biomass Valorization 2019, 10, 2703–2712. [Google Scholar] [CrossRef]
- Caetano, N.S.; Caldeira, D.; Martins, A.A.; Mata, T.M. Valorisation of Spent Coffee Grounds: Production of Biodiesel via Enzymatic Catalysis with Ethanol and a Co-solvent. Waste Biomass Valorization 2017, 8, 1981–1994. [Google Scholar] [CrossRef]
- Mota, D.A.; Rajan, D.; Heinzl, G.C.; Osório, N.; Gominho, J.; Krause, L.C.; Soares, C.M.F.; Nampoothiri, K.M.; Sukumaran, R.K.; Ferreira-Dias, S. Production of low-calorie structured lipids from spent coffee grounds or olive pomace crude oils catalyzed by immobilized lipase in magnetic nanoparticles. Bioresour. Technol. 2020, 307, 123223. [Google Scholar] [CrossRef]
- Akoh, C.C. Structured Lipids. In Food Lipids: Chemistry, Nutrition, and Biotechnology; Akoh, C.C., Min, D.B., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2002; ISBN 9781498744874. [Google Scholar]
- Kim, B.H.; Akoh, C.C. Recent Research Trends on the Enzymatic Synthesis of Structured Lipids. J. Food Sci. 2015, 80, C1713–C1724. [Google Scholar] [CrossRef]
- Ferreira-Dias, S.; Osório, N.M.; Tecelão, C. Lipase-Catalyzed Synthesis of Structured Lipids at Laboratory Scale. In Lipases and Phospholipases: Methods and Protocols, Methods in Molecular Biology; Springer: New York, NY, USA, 2018; Volume 1835, pp. 315–336. ISBN 9781493986729. [Google Scholar]
- Ferreira-Dias, S.; Natália, M.; Osório, J.R.; Tecelão, C. Structured Lipids for Foods. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 3, pp. 357–369. ISBN 9780128140260. [Google Scholar]
- Smith, R.E.; Finley, J.W.; Leveille, G.A. Overview of SALATRIM, a Family of Low-Calorie Fats. J. Agric. Food Chem. 1994, 42, 432–434. [Google Scholar] [CrossRef]
- Costa, C.M.; Osório, N.M.; Canet, A.; Rivera, I.; Sandoval, G.; Valero, F.; Ferreira-Dias, S. Production of MLM Type Structured Lipids From Grapeseed Oil Catalyzed by Non-Commercial Lipases. Eur. J. Lipid Sci. Technol. 2018, 120, 1700320. [Google Scholar] [CrossRef]
- Feltes, M.M.C.; De Oliveira Pitol, L.; Correia, J.F.G.; Grimaldi, R.; Blockc, J.M.; Ninow, J.L. Incorporation of medium chain fatty acids into fish oil triglycerides by chemical and enzymatic interesterification. Grasas y Aceites 2009, 60, 168–176. [Google Scholar] [CrossRef] [Green Version]
- Tecelão, C.; Perrier, V.; Dubreucq, E.; Ferreira-Dias, S. Production of Human Milk Fat Substitutes by Interesterification of Tripalmitin with Ethyl Oleate Catalyzed by Candida parapsilosis Lipase/Acyltransferase. J. Am. Oil Chem. Soc. 2019, 96, 777–787. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, S.; Jun, W.; Oi, L.; Lai, M.; Ping, C.; Yong, T. Production of Structured Triacylglycerol via Enzymatic Interesteri fi cation of Medium-Chain Triacylglycerol and Soybean Oil Using a Pilot-Scale Solvent-Free Packed Bed Reactor. J. Am. Oil. Chem. Soc. 2020, 97, 271–280. [Google Scholar] [CrossRef]
- Pinheiro, B.B.; Rios, N.S.; Rodríguez Aguado, E.; Fernandez-Lafuente, R.; Freire, T.M.; Fechine, P.B.A.; dos Santos, J.C.S.; Gonçalves, L.R.B. Chitosan activated with divinyl sulfone: A new heterofunctional support for enzyme immobilization. Application in the immobilization of lipase B from Candida antarctica. Int. J. Biol. Macromol. 2019, 130, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.A.; de Castro, H.F.; de Giordano, R.L.C. Triagem de suportes orgânicos e protocolos de ativação na imobilização e estabilização de lipase de Thermomyces lanuginosus. Quim. Nova 2013, 36, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Santos, M.P.F.; Brito, M.J.P.; Junior, E.C.S.; Bonomo, R.C.F.; Veloso, C.M. Pepsin immobilization on biochar by adsorption and covalent binding, and its application for hydrolysis of bovine casein. J. Chem. Technol. Biotechnol. 2019, 94, 1982–1990. [Google Scholar] [CrossRef]
- Pandey, D.; Daverey, A.; Arunachalam, K. Biochar: Production, Properties and Emerging role as a Support for Enzyme Immobilization. J. Clean. Prod. 2020, 255, 120267. [Google Scholar] [CrossRef]
- Martins, S.R.S.; Dos Santos, A.; Fricks, A.T.; Lima, Á.S.; Mattedi, S.; Silva, D.P.; Soares, C.M.F.; Cabrera-Padilla, R.Y. Protic ionic liquids influence on immobilization of Lipase Burkholderia cepacia on hybrid supports. J. Chem. Technol. Biotechnol. 2017, 92, 633–641. [Google Scholar] [CrossRef]
- Mota, D.A.; Lopes, C.; Souza, R.L.; Lima, A.S.; Krause, L.; Soares, C.M.F.; Ferreira-Dias, S. Valorization of crude oil from spent coffee grounds for the production of dietetic structured lipids catalyzed by immobilized lipases. In Proceedings of the BIOTRANS 2019, Groningen, The Netherlands, 7–11 July 2019; p. 365. [Google Scholar]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analysis. Assoc. Off. Agric. Chem. 1990, 1. [Google Scholar]
- Commission Regulation EEC No 1348/2013. on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Eur. Comm. 2013, L 338, 31–67.
- Soares, C.M.F.; De Castro, H.F.; De Moraes, F.F.; Zanin, G.M. Characterization and utilization of Candida rugosa lipase immobilized on controlled pore silica. Appl. Biochem. Biotechnol. Part A Enzym. Eng. Biotechnol. 1999, 77–79, 745–757. [Google Scholar] [CrossRef]
- European Standard Fat and Oil Derivatives—Fatty Acid Methyl Esters (FAME)—Determination of Free and Total Glycerol and Mono-, Di-, Triglyceride Contents; EN 14105; European Committee for Standardization: Brussels, Belgium, 2011; pp. 1–26.
- Cruz, R.; Cardoso, M.M.; Fernandes, L.; Oliveira, M.; Mendes, E.; Baptista, P.; Morais, S.; Casal, S. Espresso coffee residues: A valuable source of unextracted compounds. J. Agric. Food Chem. 2012, 60, 7777–7784. [Google Scholar] [CrossRef] [PubMed]
- Kovalcik, A.; Obruca, S.; Marova, I. Valorization of Spent Coffee Grounds: A review. Food Bioprod. Process. 2018, 110, 104–119. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Alves, R.C.; Oliveira, M.B.P.P. Coffee Silverskin: A Review on Potential Cosmetic Applications. Cosmetics 2018, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Efthymiopoulos, I.; Hellier, P.; Ladommatos, N.; Kay, A.; Mills-Lamptey, B. Effect of Solvent Extraction Parameters on the Recovery of Oil From Spent Coffee Grounds for Biofuel Production. Waste Biomass Valorization 2019, 10, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Al-hamamre, Z.; Foerster, S.; Hartmann, F.; Kröger, M.; Kaltschmitt, M. Oil extracted from spent coffee grounds as a renewable source for fatty acid methyl ester manufacturing. Fuel 2012, 96, 70–76. [Google Scholar] [CrossRef]
- Sonntag, N.O.V. New developments in the fatty acid industry. J. Am. Oil Chem. Soc. 1979, 56, 861A–864A. [Google Scholar] [CrossRef]
- Araújo, M.N.; Azevedo, A.Q.P.L.; Hamerski, F.; Voll, F.A.P.; Corazza, M.L. Enhanced extraction of spent coffee grounds oil using high-pressure CO2 plus ethanol solvents. Ind. Crops Prod. 2019, 141, 111723. [Google Scholar] [CrossRef]
- Tecelão, C.; Guillén, M.; Valero, F.; Ferreira-Dias, S. Immobilized heterologous Rhizopus oryzae lipase: A feasible biocatalyst for the production of human milk fat substitutes. Biochem. Eng. J. 2012, 67, 104–110. [Google Scholar] [CrossRef]
- Xu, X. Engineering of enzymatic reactions and reactors for lipid modification and synthesis. Eur. J. Lipid Sci. Technol. 2003, 105, 289–304. [Google Scholar] [CrossRef]
- Carvalho, N.B.; Lima, Á.S.; Soares, C.M.F. Use of Modified Silicas for Lipase Immobilization. Quim. Nova 2015, 38, 399–409. [Google Scholar] [CrossRef]
- Willett, S.A.; Akoh, C.C. Application of Taguchi Method in the Enzymatic Modification of Menhaden Oil to Incorporate Capric Acid. J. Am. Oil Chem. Soc. 2018, 95, 299–311. [Google Scholar] [CrossRef]
- Nunes, P.A.; Pires-Cabral, P.; Ferreira-Dias, S. Production of olive oil enriched with medium chain fatty acids catalysed by commercial immobilised lipases. Food Chem. 2011, 127, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, H.; Khan, M.K.A.; Hassan, M.I.; Islam, A.; Moosavi-Movahedi, A.A.; Ahmad, F. Evidence of non-coincidence of normalized sigmoidal curves of two different structural properties for two-state protein folding/unfolding. J. Chem. Thermodyn. 2013, 58, 351–358. [Google Scholar] [CrossRef]
- Sadana, A. A deactivation model involving pH for immobilized and soluble enzymes. Biotechnol. Lett. 1979, 1, 465–470. [Google Scholar] [CrossRef]
- Tecelão, C.; Rivera, I.; Sandoval, G.; Ferreira-Dias, S. Carica papaya latex: A low-cost biocatalyst for human milk fat substitutes production. Eur. J. Lipid Sci. Technol. 2012, 114, 266–276. [Google Scholar] [CrossRef]




| Component (%) | CS (This Study) | SCG (This Study) | CS [10] | SCG [40] |
|---|---|---|---|---|
| Protein | 15.8 ± 0.9 | 11.6 ± 1.2 | 19 ± 0.3 | 14.2 ± 0.7 |
| Fat | 3.9 ± 0.2 | 13.8 ± 1.1 | 2.4 ± 0.1 | 12.5 ± 1.6 |
| Carbohydrates | 60.4 ± 4 | 1.6 ± 0.7 | 62.2 ± 0.4 | - |
| Ash | 7.9 ± 3.2 | 0.4 ± 0.2 | 8.3 ± 0.04 | 2.0 ± 0.7 |
| Moisture | 8 ± 0.9 | 72.6 ± 0.4 | 4.8 ± 0.1 | 65.0 ± 3.0 |
| Parameter | SCG Oil | CS Oil |
|---|---|---|
| Free fatty acids (%, w/w) | 4.6 ± 0.3 | 6.75 ± 0.2 |
| K232 | 0.185 ± 0.003 | 0.257 ± 0.004 |
| K270 | 0.229 ± 0.005 | 0.329 ± 0.003 |
| Fatty Acid Group | Fatty Acid | SCG Oil (%) | CS Oil (%) |
|---|---|---|---|
| Saturated fatty acids (SFA) | Palmitic (C16:0) | 32.8 | 34.4 |
| Stearic (C18:0) | 10.9 | 9.3 | |
| Arachidic (C20:0) | 5.6 | 7.6 | |
| Behenic (C22:0) | 2.8 | 8.9 | |
| Monounsaturated fatty acids (MUFA) | Oleic (C18:1) | 11.1 | 8.3 |
| Polyunsaturated fatty acids (PUFA) | Linoleic (C18:2) | 36.8 | 31.5 |
| Σ SFA | 52.1 | 60.2 | |
| Σ MUFA | 11.1 | 8.3 | |
| Σ PUFA | 36.8 | 31.5 |
| Biocatalysts | Relative Hydrolytic Activity (%) | |
|---|---|---|
| ROL-AMB | 57 | |
| PA | ROL-SIL | 64 |
| ROL-BIO | 65 | |
| ROL-HB | 100 | |
| ROL-AMB | 67 | |
| CR | ROL-SIL | 65 |
| ROL-BIO | 63 | |
| ROL-HB | 44 | |
| New TAG Yield | ||||
|---|---|---|---|---|
| System | Model | Model Equation | R2 | Half-Life Time (h) |
| C8:0 | First-order | 0.934 | 47 | |
| C10:0 | First-order | 0.929 | 54 | |
| C8:0 Ethyl | Logistic model | 0.877 | Not attained | |
| C10:0 Ethyl | Logistic model | 0.884 | Not attained | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mota, D.A.; Santos, J.C.B.; Faria, D.; Lima, Á.S.; Krause, L.C.; Soares, C.M.F.; Ferreira-Dias, S. Synthesis of Dietetic Structured Lipids from Spent Coffee Grounds Crude Oil Catalyzed by Commercial Immobilized Lipases and Immobilized Rhizopus oryzae Lipase on Biochar and Hybrid Support. Processes 2020, 8, 1542. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8121542
Mota DA, Santos JCB, Faria D, Lima ÁS, Krause LC, Soares CMF, Ferreira-Dias S. Synthesis of Dietetic Structured Lipids from Spent Coffee Grounds Crude Oil Catalyzed by Commercial Immobilized Lipases and Immobilized Rhizopus oryzae Lipase on Biochar and Hybrid Support. Processes. 2020; 8(12):1542. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8121542
Chicago/Turabian StyleMota, Danyelle A., Jefferson C. B. Santos, Diana Faria, Álvaro S. Lima, Laiza C. Krause, Cleide M. F. Soares, and Suzana Ferreira-Dias. 2020. "Synthesis of Dietetic Structured Lipids from Spent Coffee Grounds Crude Oil Catalyzed by Commercial Immobilized Lipases and Immobilized Rhizopus oryzae Lipase on Biochar and Hybrid Support" Processes 8, no. 12: 1542. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8121542