Impact of Fermentation Processes on the Bioactive Profile and Health-Promoting Properties of Bee Bread, Mead and Honey Vinegar
Abstract
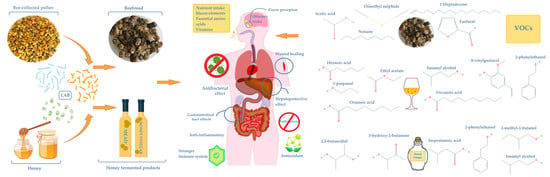
:1. Introduction
2. Bee Bread Production and Volatile Compounds
2.1. Bee Bread Fermentation Process, Yeasts Used, and Production
2.2. Volatile Organic Compounds Found in Bee Bread
3. Honey Wine (Mead) Production and Volatile Compounds
3.1. Honey Wine Fermentation Process, Yeasts Used, and Production
3.2. Volatile Organic Compounds Found in Honey Wine (Mead)
4. Honey Vinegar Production and Volatile Compounds
4.1. Honey Vinegar Fermentation Process, Yeasts Used, and Production
4.2. Volatile Organic Compounds Found in Honey Vinegar
5. The Effects of LAB from Bee Products on Human Health
5.1. Health Benefits of Bee Bread
5.1.1. Potential Probiotic Use of LAB from BB
5.1.2. Anti-Cancer Effects
5.1.3. Anti-Allergic Activity
5.1.4. Gastrointestinal Tract Effects and Gut Microbiota Modulation
5.1.5. Antibacterial and Antifungal Activity
5.1.6. Hepato-Protective Effects
5.2. Health Benefits of Honey Wine (Mead)
5.2.1. Anti-Viral Activity
5.2.2. Gastrointestinal Tract Effects
5.2.3. Wound Healing
5.2.4. Antimicrobial Activity
5.2.5. Anti-Cancer Effects
5.2.6. Antioxidant and Anti-Inflammatory Effects
5.3. Health Benefits of Honey Vinegar
5.3.1. Anti-Cancer Effect
5.3.2. Cardio-Protective Effect
5.3.3. Anti-Atherogenic Effect
5.3.4. Anti-Inflammatory Effect
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Karaçil, M.; Tek, N. Dünyada Üretilen Fermente Ürünler: Tarihsel Süreç ve Sağlık ile İlişkileri. Uludag. Üniv. Ziraat Fak. Derg. 2013, 27, 163–174. [Google Scholar]
- Balogu, T.V.; Towobola, O. Production and quality analysis of wine from honey and coconut milk blend using Saccharomyces cerevisiae. Fermentation 2017, 3, 16. [Google Scholar] [CrossRef]
- Steinkraus, K.H. Fermentations in world food processing. Comp. Rev. Food Sci. Food Saf. 2002, 1, 23–32. [Google Scholar] [CrossRef]
- Yılmaz, L. Yoğurt Benzeri Fermente Süt Ürünleri Üretiminde Farklı Probiyotik Kültür Kombinasyonlarının Kullanımı; Uludağ Üniversitesi Fen Bilimleri Enstitüsü: Doktora Tezi, Bursa, 2006. [Google Scholar]
- Erginkaya, Z.; Turhan, E.Ü.; Özer, E.A. Nohut mayalı ekmek üretimi ve hakim mikroflora. Uludağ Üniversitesi Ziraat Fakültesi Derg. 2016, 30, 89–99. [Google Scholar]
- Akbaş, Ş.; Coşkun, H. Tarhana üretimi ve özellikleri üzerine bir değerlendirme. Türkiye 2006, 9, 24–26. [Google Scholar]
- Yücel, B.; Topal, E.; Kosoglu, M. Bee products as functional food. In Superfood and Functional Food—An Overview of Their Processing and Utilization; Viduranga, W., Naofumi, S., Eds.; InTech: Rijecka, Croatia, 2017; pp. 16–33. [Google Scholar]
- Elzeini, H.M.; Ali, A.R.A.A.; Nasr, N.F.; Elenany, Y.E.; Hassan, A.A.M. Isolation and identification of lactic acid bacteria from the intestinal tracts of honey bees, Apis mellifera L., in Egypt. J. Apic. Res. 2020, 1–9. [Google Scholar] [CrossRef]
- Olofsson, T.C.; Vásquez, A. Detection and identification of a novel lactic acid bacterial flora within the honey stomach of the honeybee Apis mellifera. Curr. Microbiol. 2008, 57, 356–363. [Google Scholar] [CrossRef]
- Vasquez, A.; Olofsson, T. The lactic acid bacteria involved in the production of bee pollen and bee bread. J. Apic. Res. 2009, 48, 189–195. [Google Scholar] [CrossRef]
- Vásquez, A.; Forsgren, E.; Fries, I.; Paxton, R.J.; Flaberg, E.; Szekely, L.; Olofsson, T.C. Symbionts as major modulators of insect health: Lactic acid bacteria and honeybees. PLoS ONE 2012, 7, e33188. [Google Scholar] [CrossRef]
- Aween, M.M.; Hassan, Z.; Muhialdin, B.J.; Noor, H.M.; Eljamel, Y.A. Evaluation on antibacterial activity of Lactobacillus acidophilus strains isolated from honey. Am. J. Appl. Sci. 2012, 9, 807. [Google Scholar] [CrossRef] [Green Version]
- Aplevicz, K.S.; Mazo, J.Z.; Ilha, E.C.; Dinon, A.Z. Isolation and characterization of lactic acid bacteria and yeasts from the Brazilian grape sourdough. Braz. J. Pharm. Sci. 2014, 50, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Khalifa, S.A.; Elashal, M.; Kieliszek, M.; Ghazala, N.E.; Farag, M.A.; Saeed, A.; Xiao, J.; Zou, X.; Khatib, A.; Göransson, U.; et al. Recent insights into chemical and pharmacological studies of bee bread. Trends Food Sci. Technol. 2020, 97, 300–316. [Google Scholar] [CrossRef]
- Nagai, T.; Nagashima, T.; Suzuki, N.; Inoue, R. Antioxidant activity and angiotensin I-converting enzyme inhibition by enzymatic hydrolysates from bee bread. Z. Für Naturforschung C. 2005, 60, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Anđelković, B.; Jevtić, G.; Marković, J.; Mladenović, M.; Peševa, V. Quality of honey bee bread collected in spring. In Proceedings of the International Symposium on Animal Science, Belgrade, Serbia, 23–25 September 2014; Faculty of Agriculture: Belgrade-Zemun, Serbia, 2014; pp. 275–277. [Google Scholar]
- Fuenmayor, C.A.; Quicazán, M.C.; Figueroa, J. Desarrollo de un suplemento nutricional mediante la fermentación en fase sólida de polen de abejas empleando bacterias ácido lácticas probióticas. Aliment. Hoy 2011, 20, 17–39. [Google Scholar]
- Vamanu, E.; Vamanu, A.; Popa, O.; Băbeanu, N. The antioxidant effect of a functional product based on probiotic biomass, pollen and honey. Sci. Pap. Anim. Sci. Biotechnol. 2010, 43, 331–336. [Google Scholar]
- DeGrandi-Hoffman, G.; Eckholm, B.J.; Huang, M.H. A comparison of bee bread made by Africanized and European honey bees (Apis mellifera) and its effects on hemolymph protein titers. Apidologie 2013, 44, 52–63. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Adaškevičiūtė, V.; Kaškonas, P.; Mickienė, R.; Maruška, A. Antimicrobial and antioxidant activities of natural and fermented bee pollen. Food Biosci. 2020, 34, 100532. [Google Scholar] [CrossRef]
- Naseron, N.A.H.; Eh Teet, S. The Fermentative Activity of Bee Bread from Trigona sp. In Yogurt Production. In Prosiding Festival Agro Makanan & Bioteknologi; Politeknik Sultan Haji Ahmad Shah (POLISAS): Kuantan, Malaysia, 2016; ISBN 978-967-0778-15-0. [Google Scholar]
- Amores-Arrocha, A.; Roldán, A.; Jiménez-Cantizano, A.; Caro, I.; Palacios, V. Evaluation of the use of multiflora bee pollen on the volatile compounds and sensorial profile of Palomino fino and Riesling white young wines. Food Res. Int. 2018, 105, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, A.; Pascale, P. Consumers’ behaviours and attitudes toward healthy food products: The case of Organic and Functional foods. In Proceedings of the 113th EAAE Seminar “A Resilient European Food Industry and Food Chain in a Challenging World”, Chania, Crete, Greece, 3–6 September 2009; pp. 1–15. [Google Scholar]
- Kaškonienė, V.; Venskutonis, P.R.; Čeksterytė, V. Composition of volatile compounds of honey of various floral origin and beebread collected in Lithuania. Food Chem. 2008, 111, 988–997. [Google Scholar] [CrossRef]
- O’Neil, M.J. (Ed.) The Merck Index—An Encyclopedia of Chemicals, Drugs, and Biologicals; Royal Society of Chemistry: Cambridge, UK, 2013; pp. 11–696. [Google Scholar]
- Furia, T.E. (Ed.) CRC Handbook of Food Additives, 2nd ed.; CRC Press Inc.: Boca Raton, FL, USA, 1980; Volume 2, p. 273. [Google Scholar]
- Valette, L.; Fernandez, X.; Poulain, S.; Lizzani-Cuvelier, L.; Loiseau, A.M. Chemical composition of the volatile extracts from Brassica oleracea L. var. botrytis ‘Romanesco’cauliflower seeds. Flavour Fragr. J. 2006, 21, 107–110. [Google Scholar] [CrossRef]
- Acree, T.E.; Barnard, J.; Cunningham, D.G. A procedure for the sensory analysis of gas chromatographic effluents. Food Chem. 1984, 14, 273–286. [Google Scholar] [CrossRef]
- Héthelyi, É.; Szarka, S.; Lemberkovics, É.; Szőke, É. SPME-GC/MS identification of aroma compounds in rose flowers. Acta Agron. Hung. 2010, 58, 283–287. [Google Scholar] [CrossRef] [Green Version]
- Patty, F. (Ed.) Industrial Hygiene and Toxicology: Volume II: Toxicology, 2nd ed.; Interscience Publishers: New York, NY, USA, 1963; p. 1740. [Google Scholar]
- Larranaga, M.D.; Lewis, R.J., Sr.; Lewis, R.A. Hawley’s Condensed Chemical Dictionary, 16th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 150–1002. [Google Scholar]
- Burdock, G.A. (Ed.) Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2010; pp. 134–1520. [Google Scholar]
- Lewis, R.J., Sr. Hawley’s Condensed Chemical Dictionary, 15th ed.; John Wiley & Sons Inc.: New York, NY, USA, 2007; pp. 137–940. [Google Scholar]
- Oliveira, J.M.; Oliveira, P.E.D.R.O.; Baumes, R.L.; Maia, M.O. Volatile and glycosidically bound composition of Loureiro and Alvarinho wines. Food Sci. Technol. Int. 2008, 14, 341–353. [Google Scholar] [CrossRef] [Green Version]
- Arena, J.M.; Drew, R.H. Poisoning-Toxicology, Symptoms, Treatments, 5th ed.; Charles, C., Ed.; Thomas Publisher: Springfield, IL, USA, 1986; p. 815. [Google Scholar]
- Kaškonienė, V.; Ruočkuvienė, G.; Kaškonas, P.; Akuneca, I.; Maruška, A. Chemometric analysis of bee pollen based on volatile and phenolic compound compositions and antioxidant properties. Food Anal. Methods 2015, 8, 1150–1163. [Google Scholar] [CrossRef]
- Willey, J.T.; Jutzi, C.; Tomasino, E. Influence of Fermentation Temperature and Nutrient Addition on Chemical and Sensory Characteristics of Traditional Honey Wine. Ann. Food Pro. Preserv. 2018, 3, 1022. [Google Scholar]
- Cavalcante da Silva, S.M.P.; de Carvalho, C.A.L.; Sodré, G.D.S.; Estevinho, L.M. Production and characterization of mead from the honey of Melipona scutellaris stingless bees. J. Inst. Brew. 2018, 124, 194–200. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, A.; Pascoal, A.; Choupina, A.B.; Carvalho, C.A.; Feás, X.; Estevinho, L.M. Developments in the fermentation process and quality improvement strategies for mead production. Molecules 2014, 19, 12577–12590. [Google Scholar] [CrossRef] [Green Version]
- Cavalieri, D.; McGovern, P.E.; Hartl, D.L.; Mortimer, R.; Polsinelli, M. Evidence for S. cerevisiae Fermentation in Ancient Wine. J. Mol. Evol. 2003, 57, S226–S232. [Google Scholar] [CrossRef] [Green Version]
- Donalies, U.E.B.; Nguyen, H.T.T.; Stahl, U.; Nevoigt, E. Improvement of Saccharomyces Yeast Strains Used in Brewing, Wine Making and Baking. Adv. Biochem. Eng. Biotechnol. 2008, 111, 67–98. [Google Scholar]
- Legras, J.L.; Merdinoglu, D.; Cornuet, J.M.; Karst, F. Bread, beer and wine: Saccharomyces cerevisiae diversity reflect human history. Mol. Ecol. 2007, 16, 2091–2102. [Google Scholar] [CrossRef]
- Chitarrini, G.; Debiasi, L.; Stuffer, M.; Ueberegger, E.; Zehetner, E.; Jaeger, H.; Robatscher, P.; Conterno, L. Volatile Profile of Mead Fermenting Blossom Honey and Honeydew Honey with or without Ribes nigrum. Molecules 2020, 25, 1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bednarek, M.; Szwengiel, A.; Flórez, A.B.; Czarnecki, Z.; Mayo, B. Effect of different starter cultures on chemical and microbial parameters of buckwheat honey fermentation. Food Microbial. 2019, 82, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.P.; Dias, T.; Andrade, J.; Ramalhosa, E.; Estevinho, L.M. Mead production: Selection and characterization assays of Saccharomyces cerevisiae strains. Food Chem. Toxicol. 2009, 47, 2057–2063. [Google Scholar] [CrossRef]
- Consonni, R.; Cagliani, L.R. Recent developments in honey characterization. RSC Adv. 2015, 5, 59696–59714. [Google Scholar] [CrossRef]
- Stanimirova, I.; Üstün, B.; Cajka, T.; Riddelova, K.; Hajslova, J.; Buydens, L.M.C.; Walczak, B. Tracing the geographical origin of honeys based on volatile compounds profiles assessment using pattern recognition techniques. Food Chem. 2010, 118, 171–176. [Google Scholar] [CrossRef]
- Tananaki, C.; Zotou, A.; Thrasyvoulou, A. Determination of 1, 2-dibromoethane, 1, 4-dichlorobenzene and naphthalene residues in honey by gas chromatography–mass spectrometry using purge and trap thermal desorption extraction. J. Chromatogr. A 2005, 1083, 146–152. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Venskutonis, P.R. Floral markers in honey of various botanical and geographic origins: A review. Compr. Rev. Food Sci. F 2010, 9, 620–634. [Google Scholar] [CrossRef]
- Steinkraus, K.H. Introduction to indigenous fermented foods. In Handbook of Indigenous Fermented Foods, 2nd ed.; Marcel Dekker, CRC Press Taylor & Francis Group: New York, NY, USA, 1995; pp. 1–5. [Google Scholar]
- Solieri, L.; Giudici, P. Vinegars of the World. In Vinegars of the World; Springer: Milano, Italy, 2009; pp. 1–16. [Google Scholar]
- Sroka, P.; Tuszyński, T. Changes in organic acid contents during mead wort fermentation. Food Chem. 2007, 104, 1250–1257. [Google Scholar] [CrossRef]
- Navrátil, M.; Sturdík, E.; Gemeiner, P. Batch and continuous mead production with pectate immobilised, ethanol-tolerant yeast. Biotechnol. Lett. 2001, 23, 977–982. [Google Scholar] [CrossRef]
- Pereira, A.P.; Mendes Ferreira, A.; Oliveira, J.M.; Estevinho, L.M.; Mendes-Faia, A. High-cell-density fermentation of Saccharomyces cerevisiae for the optimisation of mead production. Food Microbiol. 2013, 33, 114–123. [Google Scholar] [CrossRef] [Green Version]
- Mendes-Ferreira, A.; Cosme, F.; Barbosa, C.; Falco, V.; Inês, A.; Mendes-Faia, A. Optimization of honey-must preparation and alcoholic fermentation by Saccharomyces cerevisiae for mead production. Int. J. Food Microbiol. 2010, 144, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Roldán, A.; Van Muiswinkel, G.C.J.; Lasanta, C.; Palacios, V.; Caro, I. Influence of pollen addition on mead elaboration: Physicochemical and sensory characteristics. Food Chem. 2010, 126, 574–582. [Google Scholar] [CrossRef]
- Ukpabi, U.J. Quality evaluation of meads produced with cassava (Manihot esculenta) floral honey under farm conditions in Nigeria. Trop. Subtrop. Agroecosyst. 2006, 6, 37–41. [Google Scholar]
- Gupta, J.K.; Sharma, R. Production technology and quality characteristics of mead and fruit-honey wines: A review. NPR 2009, 8, 345–355. [Google Scholar]
- Güçer, Y.; Güleç, H.A.; Güven, A. Bal Şarabı Üretimi. In Proceedings of the Türkiye 10, Gıda Kongresi, Erzurum, Turkey, 21–23 May 2008; pp. 315–318. [Google Scholar]
- McConnell, D.S.; Schramm, K.D. Mead success: Ingredients, processes and techniques. Zymurgy Spring 1995, 4, 33–39. [Google Scholar]
- Mendes-Ferreira, C.; Barbosa, P.; Lage, A.; Mendes-Faia, A. The impact of nitrogen on yeast fermentation and wine quality. Ciência Téc. Vitiv. 1995, 26, 17–32. [Google Scholar]
- Ramalhosa, E.; Gomes, T.; Pereira, A.P.; Dias, T.; Estevinho, L.M. Mead production: Tradition versus modernity. In Advances in Food and Nutrition Research; Academic Press: Burlington, VT, USA, 2011; Volume 63, pp. 101–118. [Google Scholar]
- Ilha, E.C.; Sant Anna, E.; Torres, R.C.; Porto, A.C.S.; Meinert, E.M. Utilization of bee (Apis mellifera) honey for vinegar production at laboratory scale. Acta Cient Venez 2000, 51, 231–235. [Google Scholar]
- Peng, B.; Li, F.; Cui, L.; Guo, Y. Effects of fermentation temperature on key aroma compounds and sensory properties of apple wine. J. Food Sci. 2015, 80, S2937–S2943. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Kahoun, D.; Řezková, S.; Královský, J. Effect of heat treatment and storage conditions on mead composition. Food Chem. 2017, 219, 357–363. [Google Scholar] [CrossRef]
- Czabaj, S.; Kawa-Rygielska, J.; Kucharska, A.Z.; Kliks, J. Effects of mead wort heat treatment on the mead fermentation process and antioxidant activity. Molecules 2017, 22, 803. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Wu, Y.L.; Lo, D.; Wu, M.C. Physicochemical property changes during the fermentation of longan (Dimocarpus longan) mead and its aroma composition using multiple yeast inoculations. J. Inst. Brew. 2013, 119, 303–308. [Google Scholar] [CrossRef]
- Pereira, A.P.; Mendes-Ferreira, A.; Oliveira, J.M.; Estevinho, L.M.; Mendes-Faia, A. Effect of Saccharomyces cerevisiae cells immobilisation on mead production. LWT Food Sci. Technol. 2014, 56, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.P.; Mendes-Ferreira, A.; Oliveira, J.M.; Estevinho, L.M.; Mendes-Faia, A. Mead production: Effect of nitrogen supplementation on growth, fermentation profile and aroma formation by yeasts in mead fermentation. J. Inst. Brew. 2015, 121, 122–128. [Google Scholar] [CrossRef] [Green Version]
- Manyi-Loh, C.E.; Ndip, R.N.; Clarke, A.M. Volatile compounds in honey: A review on their involvement in aroma, botanical origin determination and potential biomedical activities. IJMS 2011, 12, 9514–9532. [Google Scholar] [CrossRef] [Green Version]
- Pereira, A.P.; Mendes-Ferreira, A.; Dias, L.G.; Oliveira, J.M.; Estevinho, L.M.; Mendes-Faia, A. Volatile composition and sensory properties of mead. Microorganisms 2019, 7, 404. [Google Scholar] [CrossRef] [Green Version]
- Pascoal, A.; Oliveira, J.M.; Pereira, A.P.; Féas, X.; Anjos, O.; Estevinho, L.M. Influence of fining agents on the sensorial characteristics and volatile composition of mead. J. Inst. Brewing 2017, 123, 562–571. [Google Scholar] [CrossRef]
- Wintersteen, C.L.; Andrae, L.M.; Engeseth, N.J. Effect of heat treatment on antioxidant capacity and flavor volatiles of mead. J. Food Sci. 2005, 70, C119–C126. [Google Scholar] [CrossRef]
- Benes, I.; Furdikova, K.; Šmogrovičová, D. Influence of Saccharomyces cerevisiae strain on the profile of volatile organic compounds of blossom honey mead. Czech J. Food Sci. 2015, 33, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, L.V.; Marcon, A.R.; Delamare, A.P.L.; Agostini, F.; Moura, S.; Echeverrigaray, S. Selection of low nitrogen demand yeast strains and their impact on the physicochemical and volatile composition of mead. J. Food Sci. Technol. 2020, 57, 2840–2851. [Google Scholar] [CrossRef]
- Šmogrovičová, D.; Nádaský, P.; Tandlich, R.; Wilhelmi, B.S.; Cambray, G. Analytical and aroma profiles of Slovak and South African meads. Czech J. Food Sci. 2012, 30, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Sun, Y. Effects of honey variety and non-Saccharomyces cerevisiae on the flavor volatiles of mead. J. Am. Soc. Brew. Chem. 2019, 77, 40–53. [Google Scholar] [CrossRef]
- Teramoto, Y.; Sato, R.; Ueda, S. Characteristics of fermentation yeast isolated from traditional Ethiopian honey wine, ogol. Afr. J. Biotechnol. 2005, 4, 160–163. [Google Scholar]
- Castro-Vázquez, L.; Díaz-Maroto, M.C.; González-Viñas, M.A.; Pérez-Coello, M.S. Differentiation of monofloral citrus, rosemary, eucalyptus, lavender, thyme and heather honeys based on volatile composition and sensory descriptive analysis. Food Chem. 2009, 112, 1022–1030. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Díaz-Maroto, M.C.; De Torres, C.; Pérez-Coello, M.S. Effect of geographical origin on the chemical and sensory characteristics of chestnut honeys. Food Res. Int. 2010, 43, 2335–2340. [Google Scholar] [CrossRef]
- Anupama, D.; Bhat, K.K.; Sapna, V.K. Sensory and physico-chemical properties of commercial samples of honey. Food Res. Int. 2003, 36, 183–191. [Google Scholar] [CrossRef]
- Acree, T.; Arn, H. Flavornet and Human Odor Space. Available online: http://www.flavornet.org (accessed on 16 April 2020).
- Patel, S.; Shibamoto, T. Effect of different strains of Saccharomyces cerevisiae on production of volatiles in Napa Gamay wine and Petite Sirah wine. J. Agric. Food Chem. 2002, 50, 5649–5653. [Google Scholar] [CrossRef]
- Rapp, A.; Versini, G. Influence of nitrogen compounds in grapes on aroma compounds of wines. In Developments in Food Science; George, C., Ed.; Elsevier: London, UK, 1995; Volume 37, pp. 1659–1694. [Google Scholar]
- Qureshi, N.; Tamhane, D.V. Production of mead by immobilized cells of Hansenula anomala. Appl. Microbiol. Biotechnol. 1987, 27, 27–30. [Google Scholar] [CrossRef]
- Qureshi, N.; Tamhane, D.V. Mead production by continuous series reactors using immobilized yeast cells. Appl. Microbiol. Biotechnol. 1986, 23, 438–439. [Google Scholar] [CrossRef]
- Pereira, A.P. Caracterização de mel com Vista à Produção de Hidromel. Ph.D. Thesis, Instituto Politécnico de Bragança, Escola Superior Agrária, Portugal, 2008; pp. 12–55. [Google Scholar]
- Zuzuarregui, A.; del Olmo, M. Analyses of stress resistance under laboratory conditions constitute a suitable criterion for wine yeast selection. Antonie Van Leeuwenhoek 2004, 85, 271–280. [Google Scholar] [CrossRef]
- Carrasco, P.; Querol, A.; del Olmo, M. Analysis of the stress resistance of commercial wine yeast strains. Arch. Microbiol. 2001, 175, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Nanda, K.; Taniguchi, M.; Ujike, S.; Ishihara, N.; Mori, H.; Ono, H.; Murooka, Y. Characterization of acetic acid bacteria in traditional acetic acid fermentation of rice vinegar (komesu) and unpolished rice vinegar (kurosu) produced in Japan. Appl. Environ. Microbiol. 2001, 67, 986–990. [Google Scholar] [CrossRef] [Green Version]
- Natera, R.; Castro, R.; de Valme García-Moreno, M.; Hernández, M.J.; García-Barroso, C. Chemometric studies of vinegars from different raw materials and processes of production. J. Agric. Food Chem. 2003, 51, 3345–3351. [Google Scholar] [CrossRef]
- Alak, G.D. Bal ve bal Sirkesinin bazı Fiziksel ve Kimyasal Özellikleri. Master’s Thesis, Pamukkale Üniversitesi Fen Bilimleri Enstitüsü, Pamukkale, Turkey, 2015; p. 99. [Google Scholar]
- Şengün, İ.Y.; Kılıç, G. Farklı sirke çeşitlerinin mikroflorası, biyoaktif bileşenleri ve sağlık üzerine etkileri. Akademik Gıda 2019, 17, 89–101. [Google Scholar] [CrossRef] [Green Version]
- Gökırmaklı, Ç.; Budak, H.N.; Güzel-Seydim, Z.B. Antimicrobial Effect of Vinegar. Turk J. Agric. Food Sci. Technol. 2019, 7, 1635–1640. [Google Scholar]
- Wei, X.; Juan, D. The Process Research on Multi Strain Symbiotic Fermentation of Honey Vinegar Beverage. Acad. Per. Farm. Prod. Proc. 2014, 2, 151. [Google Scholar]
- Ozturk, I.; Caliskan, O.Z.N.U.R.; Tornuk, F.; Ozcan, N.; Yalcin, H.; Baslar, M.; Sagdic, O. Antioxidant, antimicrobial, mineral, volatile, physicochemical and microbiological characteristics of traditional home-made Turkish vinegars. LWT Food Sci. Technol. 2015, 63, 144–151. [Google Scholar] [CrossRef]
- Yu, Y.J.; Lu, Z.M.; Yu, N.H.; Xu, W.; Li, G.Q.; Shi, J.S.; Xu, Z.H. HS-SPME/GC-MS and chemometrics for volatile composition of Chinese traditional aromatic vinegar in the Zhenjiang region. J. Inst. Brew. 2012, 118, 133–141. [Google Scholar] [CrossRef]
- Pizarro, C.; Esteban-Díez, I.; Sáenz-González, C.; González-Sáiz, J.M. Vinegar classification based on feature extraction and selection from headspace solid-phase microextraction/gas chromatography volatile analyses: A feasibility study. Anal. Chim. Acta 2008, 608, 38–47. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Z.; Wu, X.; Yan, W. Analysis of aroma components in Sapium discolor honey vinegar by GC/MS. China Condiment 2011, 4, 89–91. [Google Scholar]
- Fariña, L.; Villar, V.; Ares, G.; Carrau, F.; Dellacassa, E.; Boido, E. Volatile composition and aroma profile of Uruguayan Tannat wines. Food Res. Int. 2015, 69, 244–255. [Google Scholar] [CrossRef]
- Leffingwell and Associates. Odor and Flavor Detection Thresholds in Water (in Parts per Billion). Available online: http://www.leffingwell.com/odorthre.htm (accessed on 29 April 2020).
- Wang, X.; Yang, S.; He, J.; Chen, L.; Zhang, J.; Jin, Y.; Zhou, J.; Zhang, Y. A green triple-locked strategy based on volatile-compound imaging, chemometrics, and markers to discriminate winter honey and Sapium honey using headspace gas chromatography-ion mobility spectrometry. Food Res. Int. 2019, 119, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Bayram, N.E.; Gerçek, Y.C.; Sorkun, K. Bee bread and bee pollen of different plant sources: Determination of phenolic content, antioxidant activity, fatty acid and element profiles. J. Food Meas. Charact. 2020, 14, 1795–1809. [Google Scholar]
- Zhao, G.; Kuang, G.; Li, J.; Hadiatullah, H.; Chen, Z.; Wang, X.; Yao, Y.; Pan, Z.-H.; Wang, Y. Characterization of aldehydes and hydroxy acids as the main contribution to the traditional Chinese rose vinegar by flavor and taste analyses. Food Res. Int. 2020, 129, 108879. [Google Scholar] [CrossRef] [PubMed]
- Bakour, M.; Fernandes, Â.; Barros, L.; Sokovic, M.; Ferreira, I.C. Bee bread as a functional product: Chemical composition and bioactive properties. LWT 2019, 109, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Mărgăoan, R.; Stranț, M.; Varadi, A.; Topal, E.; Yücel, B.; Cornea-Cipcigan, M.; Campos, M.G.R.; Vodnar, D.C. Bee collected pollen and bee bread: Bioactive constituents and health benefits. Antioxidants 2019, 8, 568. [Google Scholar] [CrossRef] [Green Version]
- Zuluaga, C.M.; Serratob, J.C.; Quicazana, M.C. Chemical, nutritional and bioactive characterization of Colombian bee-bread. Chem. Eng. 2015, 43, 175–180. [Google Scholar]
- Silici, S.; Kaplan, M.; Karaoglu, O.; Eroğlu, N. Essential and Omega Fatty Acid Content of Bee Bread and Its Effect on Heart Diseases; Apimedica & Apiquality: Roma, Italy, 2016; pp. 22–25. [Google Scholar]
- Hudz, N.; Korzeniowska, K.; Wieczorek, P.P.; Schubertová, Z.; Brindza, J.; Ivanišová, E. Approaches to the Identification and Assay of Flavonoids in Bee Bread Extracts by Spectrophotometric Method. Agrobiodiversity İmprov. Nutr. Health Life Qual. 2017, 1, 168–173. [Google Scholar]
- Urcan, A.C.; Mărghitaș, L.; Dezmirean, D.S.; Bobiș, O.; Bonta, V.; Mureșan, C.I.; Măgăoan, R. Chemical composition and biological activities of beebread—Review. BUSAMV Cluj-Napoca Anim. Sci. Biotechnol. 2017, 74, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Di Cagno, R.; Filannino, P.; Cantatore, V.; Gobbetti, M. Novel solid-state fermentation of bee-collected pollen emulating the natural fermentation process of bee bread. Food Microbiol. 2019, 82, 218–230. [Google Scholar] [CrossRef]
- Loper, G.M.; Standifer, L.N.; Thompson, M.J.; Gilliam, M. Biochemistry and microbiology of bee-collected almond (Prunus dulcis) pollen and bee bread. I-Fatty Acids, Sterols, Vitamins and Minerals. Apidologie 1980, 11, 63–73. [Google Scholar] [CrossRef] [Green Version]
- Urcan, A.C.; Criste, A.D.; Dezmirean, D.S.; Mărgăoan, R.; Caeiro, A.; Graça Campos, M. Similarity of data from bee bread with the same taxa collected in India and Romania. Molecules 2018, 23, 2491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammes, W.P.; Hertel, C. The genera lactobacillus and carnobacterium. Prokaryotes 2006, 4, 320–403. [Google Scholar]
- Klaenhammer, T.; Altermann, E.; Arigoni, F.; Bolotin, A.; Breidt, F.; Broadbent, J.; Cano, R.; Chaillou, S.; Deutscher, J.; Gasson, M.; et al. Discovering lactic acid bacteria by genomics. In Lactic Acid Bacteria: Genetics, Metabolism and Applications; Siezen, R.J., Kok, J., Abee, T., Schasfmsa, G., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 29–58. [Google Scholar]
- Servin, A.L. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbial. Rev. 2004, 28, 405–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ventura, M.; O’flaherty, S.; Claesson, M.J.; Turroni, F.; Klaenhammer, T.R.; Van Sinderen, D.; O’toole, P.W. Genome-scale analyses of health-promoting bacteria: Probiogenomics. Nat. Rev. Microbiol. 2009, 7, 61–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeyaprakash, A.; Hoy, M.A.; Allsopp, M.H. Bacterial diversity in worker adults of Apis mellifera capensis and Apis mellifera scutellata (Insecta: Hymenoptera) assessed using 16S rRNA sequences. J. Invertebr. Pathol. 2003, 84, 96–103. [Google Scholar] [CrossRef]
- Maarit, M. Lactic Acid Bacteria in Vegetable Fermentations. Food Sci. Technol. N. Y. Marcel Dekker 2004, 139, 419–430. [Google Scholar]
- Champagne, C.P.; Ross, R.P.; Saarela, M.; Hansen, K.F.; Charalampopoulos, D. Recommendations for the viability assessment of probiotics as concentrated cultures and in food matrices. Int. J. Food Microbiol. 2011, 149, 185–193. [Google Scholar] [CrossRef]
- Gorbach, S.; Seppo, S.; Yuan-Kun, L.; Benno, Y. Human Studies on Probiotics: What is scientifically proven today? In Lactic Acid Bacteria; CRC Press Taylor & Francis Group: New York, NY, USA, 2004; pp. 527–542. [Google Scholar]
- Anderson, K.E.; Sheehan, T.H.; Mott, B.M.; Maes, P.; Snyder, L.; Schwan, M.R.; Walton, A.; Jones, B.M.; Corby-Harris, V. Microbial ecology of the hive and pollination landscape: Bacterial associates from floral nectar, the alimentary tract and stored food of honey bees (Apis mellifera). PLoS ONE 2013, 8, e83125. [Google Scholar] [CrossRef] [Green Version]
- Corby-Harris, V.; Maes, P.; Anderson, K.E. The bacterial communities associated with honey bee (Apis mellifera) foragers. PLoS ONE 2014, 9, e95056. [Google Scholar] [CrossRef] [Green Version]
- Asama, T.; Arima, T.H.; Gomi, T.; Keishi, T.; Tani, H.; Kimura, Y.; Tatefuji, T.; Hashimoto, K. Lactobacillus kunkeei YB 38 from honeybee products enhances IgA production in healthy adults. J. Appl. Microbiol. 2015, 119, 818–826. [Google Scholar] [CrossRef]
- Forsgren, E.; Olofsson, T.C.; Váasquez, A.; Fries, I. Novel lactic acid bacteria inhibiting Paenibacillus larvae in honey bee larvae. Apidologie 2010, 41, 99–108. [Google Scholar] [CrossRef] [Green Version]
- Endo, A.; Salminen, S. Honeybees and beehives are rich sources for fructophilic lactic acid bacteria. Syst. Appl. Microbiol. 2013, 36, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Vamanu, A.; Vamanu, E.; Popa, O.; Câmpeanu, G.; Albulescu, R.; Drugulescu, M.; Niţã, S.; Bâbeani, N. Obtaining of a Symbiotic Product Based on Lactic Bacteria, Pollen and Honey. Pak. J. Biol. Sci. 2008, 11, 613–617. [Google Scholar] [PubMed] [Green Version]
- Huang, H.Y.; Korivi, M.; Tsai, C.H.; Yang, J.H.; Tsai, Y.C. Supplementation of Lactobacillus plantarum K68 and fruit-vegetable ferment along with high fat-fructose diet attenuates metabolic syndrome in rats with insulin resistance. Evid Based Compl. Alt. 2013, 2013, 1–12. [Google Scholar]
- De Vuyst, L.; De Vin, F.; Vaningelgem, F.; Degeest, B. Recent developments in the biosynthesis and applications of heteropolysaccharides from lactic acid bacteria. Int. Dairy J. 2001, 11, 687–707. [Google Scholar] [CrossRef]
- Jolly, L.; Vincent, S.J.F.; Duboc, P.; Neeser, J.-R. Exploiting exopolysaccharides from lactic acid bacteria. Antonie Van Leeuwenhoek 2002, 82, 367–374. [Google Scholar] [CrossRef]
- Jones, S.; Versalovic, J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol. 2009, 9, 35. [Google Scholar] [CrossRef] [Green Version]
- Almeida, C.C.; Lorena, S.L.S.; Pavan, C.R.; Akasaka, H.M.I.; Mesquita, M.A. Beneficial effects of long-term consumption of a probiotic combination of Lactobacillus casei Shirota and Bifidobacterium breve Yakult may persist after suspension of therapy in lactose-intolerant patients. Nutr. Clin. Pract. 2012, 27, 247–251. [Google Scholar] [CrossRef]
- Ceren, A.K.A.L.; Yetişmeyen, A. Probiyotik Ve Prebiyotik Tüketiminin Laktoz Intoleransi Üzerine Etkileri. Gıda 2020, 45, 380–389. [Google Scholar]
- Park, J.S.; Choi, J.W.; Jhun, J.; Kwon, J.Y.; Lee, B.I.; Yang, C.W.; Cho, M.L. Lactobacillus acidophilus improves intestinal inflammation in an acute colitis mouse model by regulation of Th17 and Treg cell balance and fibrosis development. J. Med. Food 2018, 21, 215–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uțoiu, E.; Matei, F.; Toma, A.; Diguță, C.F.; Ștefan, L.M.; Mănoiu, S. Bee collected pollen with enhanced health benefits, produced by fermentation with a Kombucha consortium. Nutrients 2018, 10, 1365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suleiman, J.B.; Bakar, A.B.A.; Mohamed, M. Malaysian Bee Bread Attenuates Apoptosis and Improves Cell Proliferation in Testis of High-Fat Diet-Induced Obese Rats. Int. J. Hum. Heal Sci. (IJHHS) 2019, 44. [Google Scholar] [CrossRef] [Green Version]
- O’sullivan, M.G.; Thornton, G.; O’sullivan, G.C.; Collins, J.K. Probiotic bacteria: Myth or reality? Trends Food Sci. Technol. 1992, 3, 309–314. [Google Scholar] [CrossRef]
- Fukushima, Y.; Li, S.-T.; Hara, H.; Terada, A.; Mitsuaoka, T. Effect of follow-up formula containing bifidobacteria (NANBF) on fecal flora and fecal metabolites in healthy children. Biosci. Microflora 1997, 16, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Ishida, Y.; Nakamura, F.; Kanzato, H.; Sawada, D.; Yamamoto, N.; Kagata, H.; Oh-Ida, M.; Takeuchi, H.; Fujiwara, S. Effect of milk fermented with Lactobacillus acidophilus strain L-92 on symptoms of Japanese cedar pollen allergy: A randomized placebo-controlled trial. Biosci. Biotechnol. Biochem. 2005, 69, 1652–1660. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, N.; Saito, T.; Uematsu, T.; Kishi, K.; Toba, M.; Kohda, N.; Suzuki, T. Oral administration of heat-killed Lactobacillus pentosus strain b240 augments protection against influenza virus infection in mice. Int. Immunopharmacol. 2011, 11, 199–203. [Google Scholar] [CrossRef]
- Izumo, T.; Ida, M.; Maekawa, T.; Furukawa, Y.; Kitagawa, Y.; Kiso, Y. Comparison of the immunomodulatory effects of live and heat-killed Lactobacillus pentosus S-PT84. J. Health Sci. 2011, 57, 304–310. [Google Scholar] [CrossRef] [Green Version]
- Bishop, N.C.; Gleeson, M. Acute and chronic effects of exercise on markers of mucosal immunity. Front. Biosci. 2009, 14, 4444–4456. [Google Scholar] [CrossRef] [Green Version]
- Fahlman, M.M.; ENGELS, H.J. Mucosal IgA and URTI in American college football players: A year longitudinal study. Med. Sci. Sport Exer. 2005, 37, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yan, Y.; Mi, J.; Zhang, H.; Lu, L.; Luo, Q.; Li, X.; Zeng, X.; Cao, Y. Simulated digestion and fermentation in vitro by human gut microbiota of polysaccharides from bee collected pollen of Chinese wolfberry. Chem. Agric. Food Chem. 2018, 66, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Misiewicz, A.; Kieliszek, M.; Czarniak, K. New Strain of Lactobacillus Delbrueckii Bacteria and Its Use for the Production of Bee Pollen. International Patent Applications WO2015093997, 25 June 2015. [Google Scholar]
- Audisio, M.C.; Terzolo, H.R.; Apella, M.C. Bacteriocin from honeybee beebread Enterococcus avium, active against Listeria monocytogenes. Appl. Environ. Microbiol. 2005, 71, 3373–3375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaškonienė, V.; Katilevičiūtė, A.; Kaškonas, P.; Maruška, A. The impact of solid-state fermentation on bee pollen phenolic compounds and radical scavenging capacity. Chem. Pap. 2018, 72, 2115–2120. [Google Scholar] [CrossRef]
- Devi, A.; Kumar, N.R.; Kaur, J. Evaluation of the antioxidative potential of Bee Products: Pollen and Bee Bread against Staphylococcus aureus Infected Balb/c mice. J. Chem. Pharm. Sci. 2016, 974, 2115. [Google Scholar]
- Shehu, M.B. The Effect of Beebread on Wound Healing in Malnourished Rabbits. Ph.D. Thesis, Universiti Sains Malaysia, Penang, Malaysia, 2014; p. 58. [Google Scholar]
- Kaur, R.; Kumar, N.R.; Harjai, K. Feedıng Bee Pollen And Bee Bread To Mıce: Effect And Antıoxıdant Status. Int. J. Therap. Applic. 2014, 18, 26–29. [Google Scholar]
- Rathore, S.; Salmerón, I.; Pandiella, S.S. Production of potentially probiotic beverages using single and mixed cereal substrates fermented with lactic acid bacteria cultures. Food Microbiol. 2012, 30, 239–244. [Google Scholar] [CrossRef]
- Fiorda, F.A.; de Melo Pereira, G.V.; Thomaz-Soccol, V.; Medeiros, A.P.; Rakshit, S.K.; Soccol, C.R. Development of kefir-based probiotic beverages with DNA protection and antioxidant activities using soybean hydrolyzed extract, colostrum and honey. LWT-Food Sci. Technol. 2016, 68, 690–697. [Google Scholar] [CrossRef]
- Asama, T.; Uematsu, T.; Kobayashi, N.; Tatefuji, T.; Hashimoto, K. Oral administration of heat-killed Lactobacillus kunkeei YB38 improves murine influenza pneumonia by enhancing IgA production. Biosci. Microb. Food Health 2017, 36, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Asama, T.; Kimura, Y.; Kono, T.; Tatefuji, T.; Hashimoto, K.; Benno, Y. Effects of heat-killed Lactobacillus kunkeei YB38 on human intestinal environment and bowel movement: A pilot study. Benef Microbes 2016, 7, 337–344. [Google Scholar] [CrossRef]
- Olofsson, T.C.; Butler, È.; Markowicz, P.; Lindholm, C.; Larsson, L.; Vásquez, A. Lactic acid bacterial symbionts in honeybees–an unknown key to honey’s antimicrobial and therapeutic activities. Int. Wound J. 2016, 13, 668–679. [Google Scholar] [CrossRef]
- Piccart, K.; Vasquez, A.; Piepers, S.; De Vliegher, S.; Olofsson, T.C. Lactic acid bacteria from the honeybee inhibit the in vitro growth of mastitis pathogens. J. Dairy Sci. 2016, 99, 2940–2944. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi, B.A.; Adetoye, A.; Ayeni, F.A. Antibacterial activities of lactic acid bacteria isolated from cow faeces against potential enteric pathogens. Afr. Health Sci. 2015, 15, 888–895. [Google Scholar] [CrossRef]
- Fonseca, M.D.S.; Santos, V.A.Q.; Calegari, G.C.; Dekker, R.F.H.; Barbosa-Dekker, A.D.M.; Cunha, M.A.A.D. Blueberry and honey vinegar: Successive batch production, antioxidant potential and antimicrobial ability. Braz. J. Food Technol. 2018, 21. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S.A. Effects of honey and its mechanisms of action on the development and progression of cancer. Molecules 2014, 19, 2497–2522. [Google Scholar] [CrossRef] [Green Version]
- Jaganathan, S.K.; Mandal, M. Antiproliferative effects of honey and of its polyphenols: A review. J. Biomed. Biotechnol. 2009, 2009, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Abubakar, M.B.; Abdullah, W.Z.; Sulaiman, S.A.; Suen, A.B. A review of molecular mechanisms of the anti-leukemic effects of phenolic compounds in honey. IJMS 2012, 13, 15054–15073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taranu, I.; Marin, D.E.; Braicu, C.; Pistol, G.C.; Sorescu, I.; Pruteanu, L.L.; Vodnar, D.C. In vitro transcriptome response to a mixture of lactobacilli strains in intestinal porcine epithelial cell line. IJMS 2018, 19, 1923. [Google Scholar] [CrossRef] [Green Version]
- Fauzi, A.N.; Norazmi, M.N.; Yaacob, N.S. Tualang honey induces apoptosis and disrupts the mitochondrial membrane potential of human breast and cervical cancer cell lines. Food Chem. Toxicol. 2011, 49, 871–878. [Google Scholar] [CrossRef]
- Ahmed, S.; Sulaiman, S.A.; Othman, N.H. Oral administration of tualang and Manuka honeys modulates breast cancer progression in Sprague-Dawley rats model. Evid Based Compl. Alt. 2017, 2017, 1–15. [Google Scholar] [CrossRef]
- Mabrouk, G.M.; Moselhy, S.S.; Zohny, S.F.; Ali, E.M.; Helal, T.E.; Amin, A.A.; Khalifa, A.A. Inhibition of methylnitrosourea (MNU) induced oxidative stress and carcinogenesis by orally administered bee honey and Nigella grains in Sprague Dawely rats. J. Exp. Clin. Cancer Res. CR 2002, 21, 341–346. [Google Scholar]
- Guerrini, S.; Mangani, S.; Romboli, Y.; Luti, S.; Pazzagli, L.; Granchi, L. Impact of Saccharomyces cerevisiae strains on health-promoting compounds in wine. Fermentation 2018, 4, 26. [Google Scholar] [CrossRef] [Green Version]
- Socha, R.; Pająk, P.; Fortuna, T.; Buksa, K. Phenolic profile and antioxidant activity of Polish meads. Int. J. Food Prop. 2015, 18, 2713–2725. [Google Scholar] [CrossRef]
- Koguchi, M.; Saigusa, N.; Teramoto, Y. Production and antioxidative activity of mead made from honey and black rice (Oryza sativa var. Indica cv. Shiun). J. Inst. Brew. 2009, 115, 238–242. [Google Scholar] [CrossRef]
- Katoh, T.; Koguchi, M.; Saigusa, N.; Teramoto, Y. Production and antioxidative activity of mead made from various types of honey and black rice (Oryza sativa var. Indica cv. Shiun). Food Sci. Technol. Res. 2011, 17, 149–154. [Google Scholar] [CrossRef]
- Diggs, L.J. Vinegar: The User Friendly Standard Text, Reference and Guide to Appreciating, Making, and Enjoying Vinegar; iUniverse: Lincoln, NE, USA, 2000; pp. 113–116. [Google Scholar]
- Felter, H.W.; Lloyd, J.U. King’s American Dispensatory, 18th ed.; Ohio Valley Co.: Cincinnati, OH, USA, 1898; Volume 3, pp. 1–4. [Google Scholar]
- Vojvodic, S.; Rehan, S.M.; Anderson, K.E. Microbial gut diversity of Africanized and European honey bee larval instars. PLoS ONE 2013, 8, e72106. [Google Scholar] [CrossRef] [Green Version]
- Crotti, E.; Rizzi, A.; Chouaia, B.; Ricci, I.; Favia, G.; Alma, A.; Bandi, C. Acetic acid bacteria, newly emerging symbionts of insects. Appl. Environ. Microbiol. 2010, 76, 6963–6970. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, Y.; Nishizawa, T.; Kouhchi, C.; Inagawa, H.; Yamaguchi, T.; Nagai, S.; Soma, G.I. Identification and characterization of lipopolysaccharide in acetic acid bacteria. Anticancer Res 2006, 26, 3997–4002. [Google Scholar]
- Hashimoto, M.; Ozono, M.; Furuyashiki, M.; Baba, R.; Hashiguchi, S.; Suda, Y.; Fujimoto, Y. Characterization of a Novel d-Glycero-d-talo-oct-2-ulosonic acid-substituted Lipid A Moiety in the Lipopolysaccharide Produced by the Acetic Acid Bacterium Acetobacter pasteurianus NBRC 3283. J. Biol. Chem. 2016, 291, 21184–21194. [Google Scholar] [CrossRef] [Green Version]
- Inagawa, H.; Kohchi, C.; Soma, G.I. Usefulness of oral administration of lipopolysaccharide for disease prevention through the induction of priming in macrophages. Anticancer Res. 2014, 34, 4497–4501. [Google Scholar]
- Kim, J.H.; Cho, H.D.; Won, Y.S.; Hong, S.M.; Moon, K.D.; Seo, K.I. Anti-Fatigue Effect of Prunus Mume Vinegar in High-Intensity Exercised Rats. Nutrients 2020, 12, 1205. [Google Scholar] [CrossRef]
- Cho, H.D.; Lee, J.H.; Jeong, J.H.; Kim, J.Y.; Yee, S.T.; Park, S.K.L.; Lee, M.-K.; Seo, K.-I. Production of novel vinegar having antioxidant and anti-fatigue activities from Salicornia herbacea L. J. Sci. Food Agric. 2016, 96, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Naseem, E.; Shamim, M.; Khan, N.I. Cardioprotective effects of herbal mixture (ginger, garlic, lemon, apple cider vinegar & honey) in experimental animal models of hyperlipidemia. Int. J. Biol. Res. 2016, 4, 28–33. [Google Scholar]
- Ishak, I.; George, P.; Ibrahim, F.W.; Yahya, H.M.; Fauzi, N.F. Acute modulatory effects of apple cider vinegar, garlic, ginger, lemon and honey mixture, with and without exercise on postprandial glycemia in non-diabetic females. J. Sains Kesihat. Malays. (Malays. J. Health Sci.) 2018, 16, 105–111. [Google Scholar]
- Yaghoobi, N.; Al-Waili, N.; Ghayour-Mobarhan, M.; Parizadeh, S.M.R.; Abasalti, Z.; Yaghoobi, Z.; Yaghoobi, F.; Esmaeili, H.; Kazemi-Bajestani, S.M.R.; Aghasizadeh, R.; et al. Natural honey and cardiovascular risk factors; effects on blood glucose, cholesterol, triacylglycerole, CRP, and body weight compared with sucrose. Sci. World J. 2008, 8, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Lucia, E.W.; Lidya, K.; Annisa, T. Efficacy of honey vinegar in hyperlipidemic rats (Rattus norvegicus). In Unity in Diversity and the Standardisation of Clinical Pharmacy Services; CRC Press Taylor & Francis Group: London, UK, 2017; pp. 139–141. [Google Scholar]
- Derakhshandeh-Rishehri, S.M.; Heidari-Beni, M.; Feizi, A.; Askari, G.R.; Entezari, M.H. Effect of honey vinegar syrup on blood sugar and lipid profile in healthy subjects. Int. J. Prev. Med. 2014, 5, 1608–1615. [Google Scholar]

| Volatile Compound | Odor Descriptor | Taste Descriptor | Odor Threshold (µg/L) | References |
|---|---|---|---|---|
| Ethanol (0.6%) (alcohol) | smoky, strong alcoholic | pungent, burning | - | [25] |
| Dimethyl sulphide (20%) | garlic, sulphurous | sulfurous, vegetative with a dairy creaminess and a slight minty after-note | 8100 | [25,26] |
| Acetic acid (13.4%) | sharp vinegar | pungent, sour, overripe fruit | 200,000 | [25] |
| Pentanonitrile (0.6%) | odorless | N/A | - | [27] |
| Dimethyl disulphide (3.0%) | garlic, sulphurous vegetable | sulfurous cabbage, malt, cream | 1200 | [28] |
| 1-Phenylpropan-2-ol (0.4%) | weak rose | sweet, pineapple | - | [29] |
| 2,4-Dimethylheptane (3.9%) | N/A | N/A | - | [24] |
| Dimethyl trisulphide (0.5%) | garlic, sulphurous, savory, meaty and vegetative with a fresh, green top-note | sulfurous, alliaceous, with a fresh, vegetative nuance | 10 | [25,28] |
| 2-octene (0.5%) | N/A | N/A | - | [24] |
| Furfural (9.8%) | sweet, woody, bready, caramellic, with a slight phenolic nuance | brown, sweet, wood, nut, caramel with a burnt astringent nuance | 710,000 | [25,26] |
| 3-Furaldehyde (0.7%) | almond, caramel, burnt sugar | N/A | - | [24] |
| 2-Hepten-1-ol (2.0%) | green | N/A | - | [24] |
| 2-Heptanone (0.4%) | cheese, fruity, green, creamy nuance | cheese, fruity, pear, coconut, waxy | 71,000 | [25,30] |
| Nonane (10.4%) | gasoline | N/A | 21,000 | [24] |
| Benzaldehyde (0.9%) | almond, fruity, powdery, nutty | sweet, almond, cherry, nutty and woody | 200 | [31,32] |
| Hexanoic acid (3.2%) | mild, fatty, cheese | sharp, acidic, cheesy, fruity notes | 300 | [25] |
| Decane (0.8%) | gasoline-like | N/A | - | [24] |
| Octanal (0.9%) | aldehydic, waxy, citrus orange with a green peely nuance | aldehyde, green with a citrus or orange note | 700 | [31,32] |
| Benzyl alcohol (0.5%) | sweet, floral, fruity with chemical nuances | sharp burning taste, fruity with balsamic nuances | 100,000 | [25,33] |
| Heptanoic acid (0.4%) | cheese, fermented, pineapple and fruity | cheese, fruity and fatty | 300,000 | [25] |
| p-Cymenene (0.3%) | spicy, coffee and nutty nuances | spicy, balsamic, musty with a nutty nuance | - | [25] |
| Undecane (1.1%) | gasoline-like to odorless | N/A | - | [24] |
| Ho-trienol (0.2%) (alcohol) | Sweet, tropical, fennel, ginger | floral, fresh, woody | - | [34] |
| Nonanal (1.2%) | waxy, citrus, with a fresh slightly green lemon peel like nuance | effervescent, citrus, cucumber, and melon rind, with raw potato and coconut like nuances | 100 | [33] |
| Benzyl nitrile (1.0%) | aromatic | N/A | - | [25] |
| Benzoic acid (1.3%) | odorless or with a slight benzaldehyde odor | slightly bitter | - | [33,35] |
| Dodecane (4.4%) | alkane | N/A | 3 | [24] |
| Decanal (0.7%) | sweet, orange, waxy and citrus coat | fatty, citrus, and orange peel with a slight melon nuance | 200 | [32] |
| 4-Ethyl-4-methyl-1-hexene (0.5%) | spice | N/A | - | [24] |
| 5-Hydroxymethylfurfural (2.5%) | fatty, butter, caramel | herbal, hay, tobacco | 770 | [25] |
| Tridecane (0.7%) | hydrocarbon | N/A | - | [24] |
| 1-Heptadecene (13.9%) | alkane | N/A | - | [24] |
| Mead Source | Strain/Fermentation Process | Major Compounds | Extraction Method | Origin | Reference |
|---|---|---|---|---|---|
| Castanea spp. and Erica spp. | S. cerevisiae Lalvin QA23; S. cerevisiae Lalvin ICV D47 | 3-methyl-1-butanol, 1-propanol, 2-phenylethanol, ethyl acetate; 4-vinylguaiacol; 4-vinylphenol; octanoic acid; decanoic acid; hexanoic acid | GC-FID and GC-MS | Portugal | [72] |
| Dark multifloral Castanea spp. and Erica spp | S. cerevisiae QA23, S. cerevisiae ICV D47 | 3-methyl-1-butanol, 4-vinylphenol, 4-vinylguaiacol, octanoic acid, hexanoic acid | GC-FID and GC–MS | Portugal | [69] |
| Dark honey (Fagopyrum spp. and/or Erica spp.) | S.cerevisiae Lalvin ICV D47 | 2-phenylethanol, acetaldehyde, acetoin, furfural, 5-hydroxymethylfurfural, ethyl lactate; ethyl acetate, octanoic acid, trans-Furan linalool oxide | GC-FID and GC-MS | Portugal | [73] |
| Erica spp. | S. cerevisiae UCD522 | Ethyl acetate; octanoic acid; hexanoic acid; decanoic acid; isoamyl alcohol | GC-MS | Portugal | [55] |
| Multifloral honey | S. cerevisiae, ENSIS-LE5 | 2-methyl butanol, 3-methyl butanol, isovaleric acid, hexanoic acid, octanoic acid | GC-FID and GC-MS | Spain | [56] |
| Buckweat (Fagopyrum esculentum) mead; Soy (Glygine max) mead | S. cerevisiae | Ethyl-butyrate, isomayl acetate, ethyl decanoate, isoamyl alcohol, 2-phenylethanol | HSPME-GC-MS | USA | [74] |
| Blossom honey (sunflower, thistle nectar) | S. cerevisiae var. bayanus MT-R1B, MM-R2, and FM-R-Fix1 | Isobutyl alcohol, Isopentyl alcohol, Ethyl acetate | SPMCE-GC | Slovakia | [75] |
| Multifloral honey | S. cerevisiae var. bayanus strains (QA23, Spark, and AWRI-R2) | 2-Phenylethanol, 2,3-Butanodiol, 3-Methyl-1- butanol, Octanoic acid | SPME with DVB/CAR/PDMS 50/30 µm fiber | Brazil | [76] |
| Acacia and Prunus honey | S. cerevisiae | Ethyl acetate, propanol | SPMCE-GC | Slovakia | [77] |
| wild natural plants of Eastern Cape (Metalasia muricata, Acacia, Eucalyptus) | Roots of succulents of the Trichodiadema genera | Ethyl acetate, propanol, i-butanol, isoamyl alcohol | SPMCE-GC | Eastern Cape in South Africa | [77] |
| Dimocarpus longan honey | S. cerevisiae 1 (IOC B 2000) | 3-Methyl-1-butanol, Isoamyl acetate, Ethyl acetate | GC | Taichung, Taiwan | [68] |
| Vitex, Acacia, Tilia, and Ziziphus honey | S. cerevisiae strain Lalvin EC1118, Torulaspora delbrueckii of ZYMAFLORE AlphaT, Kluyveromyces thermotolerans | Isopentanol, a-Phenylethyl alcohol, Ethyl Acetate, Ethyl octanoate | HSPME-GC-MS | China (Linyi and Tonghua) | [78] |
| Mandarin orange (Citrus reticulata) honey | S. cerevisiae W4, S. cerevisiae ET99, S. cerevisiae K7 | 2-Methylpropanol, 3-Methylbutanol, Propan-1-ol, Ethyl acetate | GC | Japan | [79] |
| Compound | M1 [72] | M2 [73] | M3 [55] | M4 [74] | M51 [75] | M52 [75] | M53 [75] | M6 [54] | M7 [69] | M8 [76] | Odor Threshold (μg·L−1) [83] | Odor Descriptor [83] | Flavor Descriptor |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohols | |||||||||||||
| Methanol | + | - | - | - | - | - | - | + | - | - | 500 | sweet, alcohol | N/A |
| 1-propanol | + | + | - | - | + | + | + | + | + | + | 830,000 | fruity, alcohol | N/A |
| 2-methyl-1-propanol | + | + | - | - | - | - | - | + | + | + | 75 | ether, wine | N/A |
| 1-octanol | - | - | - | - | + | + | + | - | - | - | 120 | jasmine, lemon, wax, green, citrus, coconut | wax, green, citrus, orange |
| 2,3-butanediol | - | - | - | - | - | - | - | - | - | + | 150,000 | fruity | N/A |
| Benzyl alcohol | - | - | - | - | - | - | - | - | - | + | 200,000 | sweet, fruity | N/A |
| 2-ethyl hexanol | - | - | - | - | - | - | - | - | - | + | 800 | floral | N/A |
| Isobutyl alcohol | - | - | - | - | + | + | - | - | - | - | 40,000 | balsam, sweet, whiskey | fusel, banana |
| 2-methyl-1-butanol | + | + | - | - | + | + | + | + | + | - | 40,000 | roasted, wine, onion, fruity | fusel, whiskey |
| 3-methyl-1-butanol | + | + | - | + | + | + | + | + | + | + | 30,000 | cheese; nail polish, herbaceous, slightly fruity, nut-like, bitter at high levels | fermented, fruity, banana, cognac |
| 1-hexanol | - | + | - | - | - | - | - | - | - | + | 8000 | herbaceous, grass | N/A |
| 1-heptanol | - | - | - | - | - | - | - | - | - | + | 300 | lemon, orange, copper | N/A |
| 1-nonanol | - | - | - | - | - | - | - | - | - | + | 600 | fruity | N/A |
| 1-dodecanol | - | - | - | - | - | - | - | - | - | + | 1000 | flowery | N/A |
| 3-ethoxy-1-propanol | + | + | - | - | - | - | - | + | + | + | 100 | fruity | N/A |
| 3-(methylthio)-1-propanol | + | - | - | - | - | - | - | + | + | + | 120 | suIfureous, onion | N/A |
| 2-phenyl ethanol | + | + | + | + | + | + | + | + | + | + | 10,000–14,000 | rose; flowery, pollen, perfume | floral, sweet, rose |
| Tyrosol | - | + | - | - | - | - | - | - | - | N/A | |||
| Esters | |||||||||||||
| ethyl acetate | + | + | + | - | + | + | + | + | + | + | 7500–12,300 | nail polish, fruity | sweet, cherry nuance |
| ethyl butyrate | + | + | + | + | + | + | + | - | + | + | 20 | fruity, butter, sweet | fresh, fruity, sweet |
| Ethyl 2-methylbutyrate | - | - | - | + | - | - | - | - | - | - | 1 | fruity, tropical notes | mango and cherry notes |
| Ethyl 3-methylbutyrate | - | - | - | + | - | - | - | - | - | - | 3 | sweet, fruity | blueberry, sweet, green |
| isoamyl acetate | + | + | + | + | - | - | - | + | + | - | 30 | banana, fruity | sweet, banana, green nuance |
| ethyl hexanoate | + | + | + | + | + | + | + | + | + | + | 5–14 | fruity, aniseed | fruit, fat |
| ethyl lactate | + | - | - | - | - | - | - | + | + | - | fruity, butter | N/A | |
| ethyl octanoate | + | + | + | + | - | - | - | + | + | + | 2–5 | fruity, apple, beer | N/A |
| ethyl decanoate | - | - | + | + | - | - | - | + | + | + | 200 | soap, nut-like | N/A |
| ethyl phenylacetate | + | - | - | - | - | - | - | + | + | + | 650 | floral honey, dark chocolate and cocoa notes | strong sweet, rose, honey and balsamic cocoa-like |
| 2-phenylethyl acetate | + | + | + | - | - | - | + | + | + | + | 250 | roses, honey | sweet, honey, flower |
| ethyl dodecanoate | + | - | - | - | - | - | - | + | - | + | 150 | sweet, wax, rummy with a creamy, floral nuance | wax, floral with a creamy and fruity nuance |
| ethyl propionate | - | - | + | - | - | - | - | - | - | - | sweet, fruity, rum, grape, pineapple | ether, fruit, sweet, wine, bubble gum, apple and grape notes | |
| Ethyl isobutyrate | - | - | + | - | - | - | - | - | - | - | sweet, fruity, rum | pungent, fruity with rum notes | |
| Ethyl butyrate | - | - | + | - | - | - | - | + | - | - | 20 | fruity, cognac, pineapple | fruity, sweet, apple |
| Volatile phenols | |||||||||||||
| 4-vinylguaiacol | + | - | - | - | - | - | - | + | + | + | 130 | clove | N/A |
| 4-vinylphenol | + | - | - | - | - | - | - | + | + | - | 180 | almond shell | N/A |
| 4-methylphenol | - | - | - | + | - | - | - | - | - | - | 1000 | phenol, narcissus | phenol |
| Volatile fatty acids | |||||||||||||
| 2-Methylpropanoic acid | - | + | - | - | - | - | - | - | - | - | rancid, soy | N/A | |
| isobutyric acid | + | - | - | - | - | - | - | + | + | - | cheese, butter | acidic, sour, cheese | |
| butanoic acid | + | + | - | - | - | - | - | + | + | - | cheese, butter, fruity notes | dairy, cream, fruity | |
| hexanoic acid | + | + | + | - | - | - | - | + | + | + | 420 | cheese, geranium, vegetable | N/A |
| octanoic acid | + | + | + | - | + | + | + | + | + | - | 500 | fat, rancid, cheese | rancid, soap, brandy |
| Heptanoic acid | - | - | - | - | - | - | - | - | - | + | 3000 | sweet, cheese | N/A |
| decanoic acid | + | + | + | - | - | - | - | + | + | + | 10,000 | fat, soap | N/A |
| Nonanic acid | - | - | - | - | - | - | - | - | - | + | 3000 | fat | N/A |
| dodecanoic acid | + | - | - | - | - | - | - | + | + | + | 1000 | fat, coconut oil | fat, wax |
| Phenylacetic acid | - | + | - | - | - | - | - | - | - | - | honey, flower | N/A | |
| acetaldehyde | + | + | - | - | + | + | + | + | + | + | 500–10,000 | fresh, fruity, must | fresh, green |
| Acetoin | - | + | - | - | - | - | - | - | - | - | 110 | butter, cream | N/A |
| Furfural | - | + | - | - | - | - | - | - | - | 770 | bread, almond, sweet | N/A | |
| Benzaldehyde | - | + | - | - | - | - | - | - | - | + | 200 | almond | N/A |
| 5-Hydroxymethylfurfural | - | + | - | - | - | - | - | - | - | - | 770 | almond, caramel, burnt sugar | N/A |
| Diethyl malate | - | + | - | - | - | - | - | - | - | - | brown sugar, sweet | N/A | |
| Monoethyl succinate | - | + | - | - | - | - | - | - | - | - | N/A | N/A | |
| Lactones and terpenes | |||||||||||||
| Pantolactone | - | + | - | - | - | - | - | - | - | - | cotton candy | N/A | |
| trans-Furan linalool oxide | - | + | - | - | - | - | - | - | - | - | flower | N/A | |
| cis-Furan linalool oxide | - | + | - | - | - | - | - | - | - | - | flower, wood | N/A | |
| Ho-trienol | - | + | - | - | - | - | - | - | - | + | 110 | linden | N/A |
| α-Terpineol | - | + | - | - | - | - | - | - | - | + | 40 | flower, sweet | N/A |
| Linalool | - | - | - | - | - | - | - | - | - | + | 50 | citrus, floral | N/A |
| Nerol | - | - | - | - | - | - | - | - | - | + | 400 | rose, lime | N/A |
| Citronellol | - | - | - | - | - | - | - | - | - | + | 400 | citrus | N/A |
| Nerolidol | - | - | - | - | - | - | - | - | - | + | 700 | rose, green, citrus | N/A |
| Volatile Compound | Odor Descriptor [28,101] | Taste Descriptor [28,101] | Odor Threshold (µg/L) [102] | References |
|---|---|---|---|---|
| Esters | ||||
| 2-phenylethyl ester | honey, sweet, rose, slight yeasty note and cocoa nuance | sweet, honey, rosy, slight green nectar note | 250 | [28,92,101,103] |
| 1-Phenylethyl acetate | green, must, berry nuances | fruity, berry, green, slightly nutty | 250 | [28,37,92,103] |
| 2,3-Diethyl-5-methylpyrazine | must, vegetable, roasted hazelnut, green | musty, toast, nut | - | [28,92,103] |
| 2-heptanone | cheese, fruity, green banana, creamy nuance | cheese, fruity, coconut, wax, green | 1,400,000 | [28,92,103] |
| n-butyl acetate | sharp, ether, fruity banana | sweet, ripe banana, tropical, candy, green note | 66,000 | [28,92,103] |
| ethyl pentanoate | sweet, fruity, acidic, green, berry | sweet, strawberry, tropical fruit | 10 | [18,101] |
| isoamyl acetate (0.6%) | sweet, banana, fruity | fruity, banana, green ripe nuance | 30 | [92,101] |
| ethyl hexanoate | sweet, fruity, pineapple, wax, green banana note | sweet, pineapple, fruity, wax, banana with a green nuance | 14 | [101,103] |
| 3-hydroxy-2-butanone (105.5 mg/L) | sweet butter, creamy, dairy, fat | cream, sweet, butter | 30,000 | [28,101] |
| ethyl decanoate (traces) | sweet, wax, apple | wax, fruity | 200 | [28,92,101,103] |
| benzyl acetate (traces) | sweet, fruity, floral | fruity, sweet, jasmine floral notes | 200,000 | [28,92,101,103] |
| ethyl phenylacetate (traces) | floral honey, rosy with dark chocolate and cocoa notes, anise and liquorice notes | strong sweet, rosy honey cocoa-like and yeasty nuances | 650,000 | [28,92,101,103] |
| 2-phenylethyl acetate (0.203%) | floral rosy, slight yeasty honey note, cocoa and balsamic nuances | honey, floral, rosy with a slight green nectar note | 250 | [28,94,103,104] |
| α-ionone (traces) | sweet, woody, floral violet iris (iris root), tropical | floral, powdery berry | - | [28,92,101,103] |
| 4-ethylguaiacol (0.087%) | spicy, clove-like with medicinal, vanilla notes | wood, spicy, vanilla | 9.5 | [28,92,101,103] |
| 4-ethylphenol (0.067%) | smoke, creosote | smoke, bacon, and ham | 605 | [28,92,101,103] |
| Alcohols | ||||
| 2-phenylethanol (9.3%) | floral, dried rose | sweet, rose, and bready | 10,000 | [28,92,101,103] |
| benzyl alcohol (traces) | sweet, rose, balsamic | Fruity, cherry, almond, bitter | 200,000 | [28,92,101,103] |
| 2-methyl-1-butanol (2.86%) | wine, onion, whiskey | whiskey | 40,000 | [28,101] |
| 3-methyl-1-butanol (2.82%) | alcoholic, pungent, cognac, fruity, banana and molasses | fermented, fruity, banana, cognac | 40,000 | [28,101] |
| 2-methyl-1-propanol (1.78%) | nail polish, wine | whiskey | 40,000 | [28,76,101] |
| 2,3-butanediol (19.7 mg/L) | fruity, cream, butter | - | - | [28,101] |
| Tyrosol | mild sweet, floral, fruity | N/A | - | [28,101] |
| Volatile Phenols | ||||
| Hydroxymethyl furfural | fatty buttery musty waxy caramellic | herbal hay tobacco | 770 | [28,69] |
| 2-furaldehyde | sweet, almond, caramel | sweet, woody, baked bread, nut, caramel | - | [28,101] |
| p-hydroxybenzoic acid | Phenolic, nutty | N/A | - | [28,101] |
| p-hydroxybenzaldehyde | nut, almond, vanilla and honey notes | creamy, must, vanilla and honey nuances | - | [69] |
| Medium chain fatty acids | ||||
| decanoic acid (0.061%) | sour, fat, citrus | soap, wax, fruity | 1000 | [71,101] |
| octanoic acid (0.625%) | wax, vegetable, cheese | soap, cheese, brandy | 500 | [71,101] |
| isopentanoic acid (17.3%) | cheese, dairy, sour, fruity | cream, fermented, sweet, wax, berry notes | - | [28,101] |
| Aldehydes | ||||
| Phenyl acetaldehyde | honey, rose, powdery, fermented, chocolate | honey, chocolate, spicy notes | 400 | [28,76,101] |
| benzaldehyde | almond, fruity, powder | sweet, cherry, nut, wood | 200 | [28,76] |
| Other compounds | ||||
| diethyl succinate (0.34%) | fruity, cooked apple, ylang-ylang | tropical, floral, passion fruit | 100,000 | [28,76] |
| Type of Mead | Total Phenolic Content (mg/mL Gallic Acid Eq.) | DPPH Radical Scavenging Activity (mM Trolox Eq.) | Reference |
|---|---|---|---|
| Commercial mead | 3102.9 | 16.0 | [58] |
| Home-brewed mead from soy honey | 163.6 | 7.1 | |
| Buckweat mead | 300.6 | 3.8 | |
| Soy mead | 167.7 | 3.4 | |
| Chinese milk vetch honey mead | 100 | 0.3 | [169] |
| Chinese milk vetch honey and polished rice mead | 100 | 0.09 | |
| Chinese milk vetch honey and black rice mead | 200 | 0.3 | |
| Buckwheat honey and black rice mead | 400 | 0.4 | |
| Buckwheat honey and polished rice mead | 300 | 0.4 | |
| Buckwheat honey mead | 300 | 0.39 | |
| Chinese milk vetch honey mead | 194 | 0.20 | [170] |
| Clover honey mead | 198 | 0.24 | |
| mixture of clover and acacia honey mead | 198 | 0.24 | |
| lemon honey mead | 210 | 0.28 | |
| acacia honey mead | 208 | 0.22 | |
| Dry mead | 2.67 | 1.54 | [38] |
| Sweet mead | 2.47 | 1.41 | |
| MBW | 419.27 | 1.01 | [67] |
| MBM | 416.97 | 1.02 | |
| MBF | 446.10 | 0.98 | |
| MPW | 250.43 | 0.63 | |
| MPM | 259.87 | 0.75 | |
| MPF | 213.57 | 0.74 | |
| HBW | 254.80 | 0.62 | |
| HBM | 269.57 | 0.86 | |
| HBF | 266.40 | 0.87 | |
| HPW | 215.67 | 0.59 | |
| HPM | 248.80 | 0.73 | |
| HPF | 177.30 | 0.83 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mărgăoan, R.; Cornea-Cipcigan, M.; Topal, E.; Kösoğlu, M. Impact of Fermentation Processes on the Bioactive Profile and Health-Promoting Properties of Bee Bread, Mead and Honey Vinegar. Processes 2020, 8, 1081. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8091081
Mărgăoan R, Cornea-Cipcigan M, Topal E, Kösoğlu M. Impact of Fermentation Processes on the Bioactive Profile and Health-Promoting Properties of Bee Bread, Mead and Honey Vinegar. Processes. 2020; 8(9):1081. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8091081
Chicago/Turabian StyleMărgăoan, Rodica, Mihaiela Cornea-Cipcigan, Erkan Topal, and Mustafa Kösoğlu. 2020. "Impact of Fermentation Processes on the Bioactive Profile and Health-Promoting Properties of Bee Bread, Mead and Honey Vinegar" Processes 8, no. 9: 1081. https://0-doi-org.brum.beds.ac.uk/10.3390/pr8091081