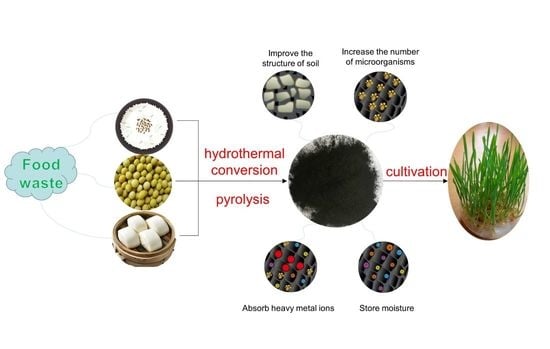
Effect of Biochar Prepared from Food Waste through Different Thermal Treatment Processes on Crop Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material Selection and Processing
2.2. Hydrothermal Conversion
2.3. Pyrolysis
2.4. Biochar Treatment
2.5. Plant Cultivation
2.6. Characterization of Biochar
2.7. Statistical Analysis
3. Results and Discussion
3.1. The Conditions of Optimum Biochar Yield for Biochar Prepared from Food Waste
3.2. Analysis of Cultivation of Wheats Using Biochar Prepared from FW
3.3. Analysis of Characterization Results of Biochar
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 709. [Google Scholar] [CrossRef] [PubMed]
- Anex, R.P.; Aden, A.; Kazi, F.K.; Fortman, J.; Swanson, R.M.; Wright, M.M.; Satrio, J.A.; Brown, R.C.; Daugaard, D.E.; Platon, A.; et al. Techno-economic comparison of biomass-to-transportation fuels via pyrolysis, gasification, and biochemical pathways. Fuel 2010, 89 (Suppl. S1), S29–S35. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, B.-J.; Huang, M.-Y.; Chang, J.-S. Thermochemical conversion of microalgal biomass into biofuels: A review. Bioresour. Technol. 2015, 184, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Ben, H.; Wu, Z.; Han, G.; Jiang, W.; Ragauskas, A. In-situ evaluation for upgrading of biomass model compounds over noble metal catalysts by isotopic tracing and NMR monitoring (vol 137, pg 253, 2019). J. Anal. Appl. Pyrolysis 2019, 142. [Google Scholar] [CrossRef]
- Ben, H.; Wu, Z.; Han, G.; Jiang, W.; Ragauskas, A. Pyrolytic Behavior of Major Biomass Components in Waste Biomass. Polymers 2019, 11, 324. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Aust. J. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Shu, T.; Lu, F.; Wang, Q.; Lu, P. Study on Pore Structure Properties of Steam Activated Biomass Chars; Springer: Berlin/Heidelberg, Germany, 2012; pp. 216–219. [Google Scholar]
- Bruun, E.W.; Ambus, P.; Egsgaard, H.; Hauggaard-Nielsen, H. Effects of slow and fast pyrolysis biochar on soil C and N turnover dynamics. Soil Biol. Biochem. 2012, 46, 73–79. [Google Scholar] [CrossRef]
- Sohi, S.P. Carbon Storage with Benefits. Science 2012, 338, 1034–1035. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1. [Google Scholar] [CrossRef] [Green Version]
- Bogusz, A.; Oleszczuk, P.; Dobrowolski, R. Application of laboratory prepared and commercially available biochars to adsorption of cadmium, copper and zinc ions from water. Bioresour. Technol. 2015, 196, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Shaaban, M.; Mehmood, S.; Zhu, J.; Fu, Q.L.; Hu, H.Q. Efficiency of C3 and C4 Plant Derived-Biochar for Cd Mobility, Nutrient Cycling and Microbial Biomass in Contaminated Soil. Bull. Environ. Contam. Toxicol. 2018, 100, 834–838. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U., Jr.; Bricka, M.; Smith, F.; Yancey, B.; Mohammad, J.; Steele, P.H.; Alexandre-Franco, M.F.; Gomez-Serrano, V.; Gong, H. Sorption of arsenic, cadmium, and lead by chars produced from fast pyrolysis of wood and bark during bio-oil production. J. Colloid Interface Sci. 2007, 310, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Sombroek, W.G.; Nachtergaele, F.O.; Hebel, A. Amounts, dynamics and sequestering of carbon in tropical and subtropical soils. Ambio 1993, 22, 417–426. [Google Scholar]
- Zhou, G.; Zhou, X.; Zhang, T.; Du, Z.; He, Y.; Wang, X.; Shao, J.; Cao, Y.; Xue, S.; Wang, H.; et al. Biochar increased soil respiration in temperate forests but had no effects in subtropical forests. For. Ecol. Manag. 2017, 405, 339–349. [Google Scholar] [CrossRef]
- Xu, L.; Fang, H.; Deng, X.; Ying, J.; Lv, W.; Shi, Y.; Zhou, G.; Zhou, Y. Biochar application increased ecosystem carbon sequestration capacity in a Moso bamboo forest. For. Ecol. Manag. 2020, 475. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Subbotina, I.; Chen, H.; Bogomolova, I.; Xu, X. Black carbon decomposition and incorporation into soil microbial biomass estimated by C-14 labeling. Soil Biol. Biochem. 2009, 41, 210–219. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Lehmann, J.; Thies, J.E.; Burton, S.D. Stability of black carbon in soils across a climatic gradient. J. Geophys. Res. Biogeosci. 2008, 113. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, M.W.I.; Noack, A.G. Black carbon in soils and sediments: Analysis, distribution, implications, and current challenges. Glob. Biogeochem. Cycles 2000, 14, 777–793. [Google Scholar] [CrossRef]
- Zhang, A.; Cui, L.; Pan, G.; Li, L.; Hussain, Q.; Zhang, X.; Zheng, J.; Crowley, D. Effect of biochar amendment on yield and methane and nitrous oxide emissions from a rice paddy from Tai Lake plain, China. Agric. Ecosyst. Environ. 2010, 139, 469–475. [Google Scholar] [CrossRef]
- Steiner, C.; Teixeira, W.G.; Lehmann, J.; Nehls, T.; de Macedo, J.L.V.; Blum, W.E.H.; Zech, W. Long term effects of manure, charcoal and mineral fertilization on crop production and fertility on a highly weathered Central Amazonian upland soil. Plant. Soil 2007, 291, 275–290. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Using poultry litter biochars as soil amendments. Aust. J. Soil Res. 2008, 46, 437–444. [Google Scholar] [CrossRef]
- Lai, C.; Yang, Y.; Tao, C.; Wang, Y.; Guo, J.; Wang, B.; Ruan, Y.; Zhao, Y. Effects of replanted banana after rotation of different crops on banana production and soil fertility quality. Jiangsu J. Agric. Sci. 2018, 34, 299–306. [Google Scholar]
- Rondon, M.A.; Lehmann, J.; Ramirez, J.; Hurtado, M. Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol. Fertil. Soils 2007, 43, 699–708. [Google Scholar] [CrossRef]
- Uzoma, K.C.; Inoue, M.; Andry, H.; Fujimaki, H.; Zahoor, A.; Nishihara, E. Effect of cow manure biochar on maize productivity under sandy soil condition. Soil Use Manag. 2011, 27, 205–212. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Steinbeiss, S.; Gleixner, G.; Antonietti, M. Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol. Biochem. 2009, 41, 1301–1310. [Google Scholar] [CrossRef]
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar induced soil microbial community change: Implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A.; et al. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529–533. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, Y.; Chen, Y.; Liu, G. Non-structural carbohydrate dynamics in Robinia pseudoacacia saplings under three levels of continuous drought stress. Trees Struct. Funct. 2015, 29, 1837–1849. [Google Scholar] [CrossRef]
- Utomo, W.; Islami, T.; Wisnubroto, E.I.; Suhartini. Biochar as a carrier for nitrogen plant nutrition: 2, The growth of maize (Zea mays L.) applied with nitrogen enriched biochar on different soil texture. Biosci. Res. 2018, 15, 1749–1756. [Google Scholar]
- Li, C.; Li, X. Effect of biochar preparation and its application amount on soil carbon pool and plant growth. J. South. Argic. 2015, 46, 1786–1791. [Google Scholar]
- Prapagdee, S.; Tawinteung, N. Effects of biochar on enhanced nutrient use efficiency of green bean, Vigna radiata L. Environ. Sci. Pollut. Res. 2017, 24, 9460–9467. [Google Scholar] [CrossRef]
- Zhou, Z.; Bu, X.; Wu, Y.; Xue, J. Research advances in biochar effects on soil microbial properties. J. Nanjing For. Univ. Nat. Sci. Ed. 2016, 40, 1–8. [Google Scholar]
- Lin, Q.; Zhang, L.; Riaz, M.; Zhang, M.; Xia, H.; Lv, B.; Jiang, C. Assessing the potential of biochar and aged biochar to alleviate aluminum toxicity in an acid soil for achieving cabbage productivity. Ecotoxicol. Environ. Saf. 2018, 161, 290–295. [Google Scholar] [CrossRef]
- Liu, Z.; Balasubramanian, R. Upgrading of waste biomass by hydrothermal carbonization (HTC) and low temperature pyrolysis (LTP): A comparative evaluation. Appl. Energy 2014, 114, 857–864. [Google Scholar] [CrossRef]
- Magdeldin, M.; Kohl, T.; De Blasio, C.; Jarvinen, M.; Park, S.W.; Giudici, R. The BioSCWG Project: Understanding the Trade-Offs in the Process and Thermal Design of Hydrogen and Synthetic Natural Gas Production. Energies 2016, 9, 838. [Google Scholar] [CrossRef] [Green Version]
- Kruse, A.; Dahmen, N. Water—A magic solvent for biomass conversion. J. Supercrit. Fluids 2015, 96, 36–45. [Google Scholar] [CrossRef]
- Kaushik, R.; Parshetti, G.K.; Liu, Z.; Balasubramanian, R. Enzyme-assisted hydrothermal treatment of food waste for co-production of hydrochar and bio-oil. Bioresour. Technol. 2014, 168, 267–274. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Q.; Xie, W.; Wang, Y.; Kang, J. Effects of temperature, time and acidity of hydrothermal carbonization on the hydrochar properties and nitrogen recovery from corn stover. Biomass Bioenergy 2019, 122, 175–182. [Google Scholar] [CrossRef]
- Yao, Z.; Ma, X. Effects of hydrothermal treatment on the pyrolysis behavior of Chinese fan palm. Bioresour. Technol. 2018, 247, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Ma, X. Hydrothermal carbonization of Chinese fan palm. Bioresour. Technol. 2019, 282, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Antal, M.J.; Gronli, M. The art, science, and technology of charcoal production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Nelson, P.F. Agronomic properties of wastewater sludge biochar and bioavailability of metals in production of cherry tomato (Lycopersicon esculentum). Chemosphere 2010, 78, 1167–1171. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 2): Conversion technologies. Bioresour. Technol. 2002, 83, 47–54. [Google Scholar] [CrossRef]
- Yao, Z.; Ma, X.; Lin, Y. Effects of hydrothermal treatment temperature and residence time on characteristics and combustion behaviors of green waste. Appl. Therm. Eng. 2016, 104, 678–686. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, L.; Xu, B.-C.; Li, F.-M. The relationship between competitive ability and yield stability in an old and a modern winter wheat cultivar. Plant. Soil 2011, 347, 7–23. [Google Scholar] [CrossRef]
- Vandeleur, R.K.; Gill, G.S. The impact of plant breeding on the grain yield and competitive ability of wheat in Australia. Aust. J. Agric. Res. 2004, 55, 855–861. [Google Scholar] [CrossRef]
- Yenish, J.R.; Young, F.L. Winter wheat competition against jointed goatgrass (Aegilops cylindrica) as influenced by wheat plant height, seeding rate, and seed size. Weed Sci. 2004, 52, 996–1001. [Google Scholar] [CrossRef]
- Mason, H.; Goonewardene, L.; Spaner, D. Competitive traits and the stability of wheat cultivars in differing natural weed environments on the northern Canadian Prairies. J. Agric. Sci. 2008, 146, 21–33. [Google Scholar] [CrossRef]
- Murphy, K.M.; Dawson, J.C.; Jones, S.S. Relationship among phenotypic growth traits, yield and weed suppression in spring wheat landraces and modern cultivars. Field Crop. Res. 2008, 105, 107–115. [Google Scholar] [CrossRef]
- Kannan, S.; Gariepy, Y.; Raghavan, G.S.V. Optimization and characterization of hydrochar produced from microwave hydrothermal carbonization of fish waste. Waste Manag. 2017, 65, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Balasubramanian, R.; Srinivasan, M.P. Hydrothermal conversion of biomass waste to activated carbon with high porosity: A review. Chem. Eng. J. 2016, 283, 789–805. [Google Scholar] [CrossRef]
- Tandy, S.; Healey, J.R.; Nason, M.A.; Williamson, J.C.; Jones, D.L.; Thain, S.C. FT-IR as an alternative method for measuring chemical properties during composting. Bioresour. Technol. 2010, 101, 5431–5436. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Li, Y.; Zhang, J.; Wu, S.; Cao, Y.; Liang, P.; Zhang, J.; Wong, M.H.; Wang, M.; Shan, S.; et al. Influence of pyrolysis temperature on properties and environmental safety of heavy metals in biochars derived from municipal sewage sludge. J. Hazard. Mater. 2016, 320, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, D.S.; Adhikari, S.; Shojaeiarani, J.; Bajwa, S.G.; Pandey, P.; Shanmugam, S.R. Characterization of bio-carbon and ligno-cellulosic fiber reinforced bio-composites with compatibilizer. Constr. Build. Mater. 2019, 204, 193–202. [Google Scholar] [CrossRef]
- Liu, Z.; Han, G. Production of solid fuel biochar from waste biomass by low temperature pyrolysis. Fuel 2015, 158, 159–165. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Hoekman, S.K.; Balasubramanian, R. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]















| Crops | Yield Increase Rate | Biochar Addition | Literature |
|---|---|---|---|
| Paddy | +12%, +14% | 10 t/hm2, 40 t/hm2 | [21] |
| Paddy and sorghum | +75% | 11 t/hm2 | [22] |
| Radish | +42%, +96% | 10 t/hm2, 50 t/hm2 | [23] |
| Perennial ryegrass | +20%, +52% | 30 t/hm2, 60 t/hm2 | [24] |
| Soybean | +46% | 90 g/kg | [25] |
| Biomass Species | Moisture Content/wt% |
|---|---|
| Rice (cooked) | 56.76 |
| Soybean (cooked) | 61.15 |
| Steamed bread (cooked) | 42.97 |
| Sample | Reactor | Temperature (℃) | Time (min) | Concentration of Sulfuric Acid (v/v%) | Biochar Yield (%) | Biochar Naming |
|---|---|---|---|---|---|---|
| Rice cooked | HPR | 210 | 60 | 3 | 41.71 ± 1.45 | RH |
| MW | 180 | 20 | 3 | 10.34 ± 0.20 | RM | |
| Soybean cooked | HPR | 180 | 30 | 0 | 20.00 ± 0.60 | SH |
| MW | 200 | 20 | 3 | 17.35 ± 0.72 | SM | |
| Steamed bread cooked | HPR | 240 | 90 | 0 | 42.56 ± 1.86 | BH |
| MW | 180 | 20 | 3 | 6.83 ± 0.64 | BM |
| The Type of Biochar | Weight of Biochar Used to Directly Cultivate Wheat [g] |
|---|---|
| RH | 2.0096 |
| RM | 2.0069 |
| RF | 2.0073 |
| BH | 2.0089 |
| BM | 2.0030 |
| BF | 2.0052 |
| SH | 2.0078 |
| SM | 2.0079 |
| SF | 2.0068 |
| S(Soil) | -- |
| W(Water) | -- |
| G(Silica) | -- |
| Sample Name | B | BF | BH | BM | R | RF |
|---|---|---|---|---|---|---|
| BET surface area (m2/g) | 0.0244 | 0.0142 | 1.0959 | 2.7996 | 0.0766 | 0.0063 |
| The height of H6 (cm) | -- | 16.40 | 10.92 | 10.81 | -- | 12.00 |
| Sample name | RH | RM | S | SF | SH | SM |
| BET surface area (m2/g) The height of H6 (cm) | 0.6856 7.82 | 2.6872 8.26 | 0.0822 -- | 0.0058 16.40 | 0.0966 13.93 | 0.1758 11.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, H.; Ben, H.; Wu, F. Effect of Biochar Prepared from Food Waste through Different Thermal Treatment Processes on Crop Growth. Processes 2021, 9, 276. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9020276
Jia H, Ben H, Wu F. Effect of Biochar Prepared from Food Waste through Different Thermal Treatment Processes on Crop Growth. Processes. 2021; 9(2):276. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9020276
Chicago/Turabian StyleJia, Hang, Haoxi Ben, and Fengze Wu. 2021. "Effect of Biochar Prepared from Food Waste through Different Thermal Treatment Processes on Crop Growth" Processes 9, no. 2: 276. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9020276