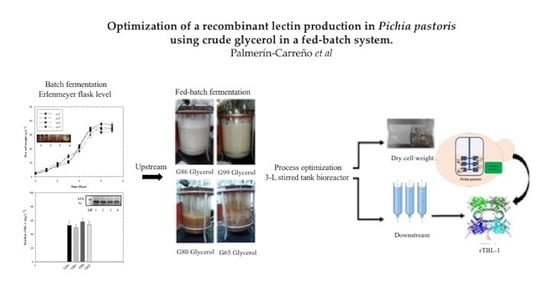
Optimization of a Recombinant Lectin Production in Pichia pastoris Using Crude Glycerol in a Fed-Batch System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strain and Chemicals
2.2. Inoculum Preparation
2.3. Fermentation Conditions
2.3.1. Optimization of Glycerol Concentration in Flask Fermentation
2.3.2. Optimization of pH in Batch Fermentation
2.3.3. Operation Parameter Optimization in Fed-Batch Fermentation
2.4. Purification of rTBL-1
2.5. rTBL-1 Identification
2.6. UHPLC-ESI-QTOF/MS Analysis
2.7. Comparison between rTBL-1 and the Native Lectin Genetic Sequence and Cytotoxicity Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effect of Glycerol Concentration on rTBL-1 Production in Batch Cultures
3.2. Comparison of the Four Purity Levels of Glycerol as Substrate in Batch Cultures
3.3. Effect of pH on rTBL-1 Production in Batch Cultures
3.4. Operation Parameter Optimization Using G65 by Fed-Batch Fermentation
3.5. Comparison of Process Parameters Using G99 and G65 by Fed-Batch Fermentation
3.6. UHPLC-ESI-QTOF/MS Analysis and Purification of rTBL-1
3.7. Comparison Between rTBL-1 and the Native Lectin and Cytotoxic Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Estrada-Martínez, L.E.; Moreno-Celis, U.; Cervantes-Jiménez, R.; Ferriz-Martínez, R.A.; Blanco-Labra, A.; García-Gasca, T. Plant Lectins as Medical Tools against Digestive System Cancers. Int. J. Mol. Sci. 2017, 18, 1403. [Google Scholar] [CrossRef] [Green Version]
- García-Gasca, T.; García-Cruz, M.; Hernandez-Rivera, E.; López-Matínez, J.; Castañeda-Cuevas, A.L.; Yllescas-Gasca, L.; Rodríguez-Méndez, A.J.; Mendiola-Olaya, E.; Castro-Guillén, J.L.; Blanco-Labra, A. Effects of Tepary Bean (Phaseolus acutifolius) Protease Inhibitor and Semipure Lectin Fractions on Cancer Cells. Nutr. Cancer 2012, 64, 1269–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Celis, U.; López-Martínez, F.J.; Cervantes-Jiménez, R.; Ferríz-Martínez, R.A.; Blanco-Labra, A.; García-Gasca, T. Tepary Bean (Phaseolus acutifolius) Lectins Induce Apoptosis and Cell Arrest in G0/G1 by P53(Ser46) Phosphorylation in Colon Cancer Cells. Mol. 2020, 25, 1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferriz-Martínez, R.; García-García, K.; Torres-Arteaga, I.; Rodriguez-Mendez, A.J.; Guerrero-Carrillo, M.D.J.; Moreno-Celis, U.; Ángeles-Zaragoza, M.V.; Blanco-Labra, A.; Gallegos-Corona, M.A.; Robles-Álvarez, J.P.; et al. Tolerability assessment of a lectin fraction from Tepary bean seeds (Phaseolus acutifolius) orally administered to rats. Toxicol. Rep. 2015, 2, 63–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno-Celis, U.; López-Martínez, J.; Blanco-Labra, A.; Cervantes-Jiménez, R.; Estrada-Martínez, L.E.; García-Pascalin, A.E.; Guerrero-Carrillo, M.D.J.; Rodríguez-Méndez, A.J.; Mejía, C.; Ferríz-Martínez, R.A.; et al. Phaseolus acutifolius Lectin Fractions Exhibit Apoptotic Effects on Colon Cancer: Preclinical Studies Using Dimethilhydrazine or Azoxi-Methane as Cancer Induction Agents. Molecules 2017, 22, 1670. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Alarcón, D.; Varrot, A.; Fitches, E.; Gatehouse, J.A.; Cao, M.; Pyati, P.; Blanco-Labra, A.; Garcia-Gasca, T. Recombinant Lectin from Tepary Bean (Phaseolus acutifolius) with Specific Recognition for Cancer-Associated Glycans: Production, Structural Characterization, and Target Identification. Biomolecules 2020, 10, 654. [Google Scholar] [CrossRef] [Green Version]
- Gellissen, G. Heterologous protein production in methylotrophic yeasts. Appl. Microbiol. Biotechnol. 2000, 54, 741–750. [Google Scholar] [CrossRef]
- Barrero, J.J.; Casler, J.C.; Valero, F.; Ferrer, P.; Glick, B.S. An improved secretion signal enhances the secretion of model proteins from Pichia pastoris. Microb. Cell Factories 2018, 17, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.; Hearn, M.T.W. Expression of heterologous proteins in Pichia pastoris: A useful experimental tool in protein engineering and production. J. Mol. Recognit. 2004, 18, 119–138. [Google Scholar] [CrossRef]
- Yin, J.; Li, G.; Ren, X.; Herrler, G. Select what you need: A comparative evaluation of the advantages and limitations of frequently used expression systems for foreign genes. J. Biotechnol. 2007, 127, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Jahic, M.; Knoblechner, J.; Charoenrat, T.; Enfors, S.-O.; Veide, A. Interfacing Pichia pastoris cultivation with expanded bed adsorption. Biotechnol. Bioeng. 2006, 93, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Kosamia, N.M.; Samavi, M.; Uprety, B.K.; Rakshit, S.K. Valorization of Biodiesel Byproduct Crude Glycerol for the Production of Bioenergy and Biochemicals. Catalysts 2020, 10, 609. [Google Scholar] [CrossRef]
- Looser, V.; Bruhlmann, B.; Bumbak, F.; Stenger, C.; Costa, M.; Camattari, A.; Fotiadis, D.; Kovar, K. Cultivation strategies to enhance productivity of Pichia pastoris: A review. Biotechnol. Adv. 2015, 33, 1177–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koizumi, J.; Aiba, S. Reassessment of the dynamic KL a method. Biotechnol. Bioeng. 1984, 26, 1131–1133. [Google Scholar] [CrossRef]
- Fuchs, R.; Ryu, D.D.Y.; Humphrey, A.E. Effect of Surface Aeration on Scale-Up Procedures for Fermentation Processes. Ind. Eng. Chem. Process. Des. Dev. 1971, 10, 190–196. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-Dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; Dezonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef]
- Packer, N.H.; Ball, M.S.; Devine, P.L.; Link, A.J. Glycoprotein Detection of 2-D Separated Proteins. 2-D Proteome Anal. Protoc. 2003, 112, 341–352. [Google Scholar] [CrossRef]
- Vega-Rojas, L.J.; Luzardo-Ocampo, I.; Mosqueda, J.; Palmerín-Carreño, D.M.; Escobedo-Reyes, A.; Blanco-Labra, A.; Escobar-García, K.; García-Gasca, T. Bioaccessibility and In Vitro Intestinal Permeability of a Recombinant Lectin from Tepary Bean (Phaseolus acutifolius) Using the Everted Intestine Assay. Int. J. Mol. Sci. 2021, 22, 1049. [Google Scholar] [CrossRef]
- Arteaga, I.T.; Guillen, J.L.C.; Mendiola-Olaya, E.; Garcia-Gasca, T.; Zaragoza, M.A.; Santoyo, V.G.; Castillo, J.A.T.; Aguirre, C.; Phinney, B.; Blanco-Labra, A. Characterization of Two Non-Fetuin-Binding Lectins from Tepary Bean (Phaseolus acutifolius) Seeds with Differential Cytotoxicity on Colon Cancer Cells. J. Glycobiol. 2016, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Preece, D.A.; Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: Hoboken, NJ, USA, 1978. [Google Scholar]
- Pal, Y.; Khushoo, A.; Mukherjee, K.J. Process optimization of constitutive human granulocyte–macrophage colony-stimulating factor (hGM-CSF) expression in Pichia pastoris fed-batch culture. Appl. Microbiol. Biotechnol. 2006, 69, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.; Belo, I.; Mota, M. Batch and fed-batch growth of Pichia pastoris under increased air pressure. Bioprocess Biosyst. Eng. 2012, 36, 1267–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, M.; Wang, Z.-Y.; Fu, J.-Y.; Li, H.-W.; Zhang, J.; Zhang, X.-F.; Luo, W.; Lv, P.-M. Crude glycerol impurities improve Rhizomucor miehei lipase production by Pichia pastoris. Prep. Biochem. Biotechnol. 2021, 1–11. [Google Scholar] [CrossRef]
- Tang, S.; Boehme, L.; Lam, H.; Zhang, Z. Pichia pastoris fermentation for phytase production using crude glycerol from biodiesel production as the sole carbon source. Biochem. Eng. J. 2009, 43, 157–162. [Google Scholar] [CrossRef]
- Wegner, E.H. Biochemical Conversions by Yeast Fermentation at High-Cell Densities. U.S. Patent 4,414,329, 8 November 1983. [Google Scholar]
- Charoenrat, T.; Khumruaengsri, N.; Promdonkoy, P.; Rattanaphan, N.; Eurwilaichitr, L.; Tanapongpipat, S.; Roongsawang, N. Improvement of recombinant endoglucanase produced in Pichia pastoris KM71 through the use of synthetic medium for inoculum and pH control of proteolysis. J. Biosci. Bioeng. 2013, 116, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Z. Engineering strategies for enhanced production of protein and bio-products in Pichia pastoris: A review. Biotechnol. Adv. 2018, 36, 182–195. [Google Scholar] [CrossRef]
- Dehnavi, E.; Siadat, S.O.R.; Roudsari, M.F.; Khajeh, K. Cloning and high-level expression of β-xylosidase from Selenomonas ruminantium in Pichia pastoris by optimizing of pH, methanol concentration and temperature conditions. Protein Expr. Purif. 2016, 124, 55–61. [Google Scholar] [CrossRef]
- Gonçalves, A.M.G.; Pedro, A.; Maia, C.; Sousa, F.; Queiroz, J.A.; Passarinha, L.A. Pichia pastoris: A Recombinant Microfactory for Antibodies and Human Membrane Proteins. J. Microbiol. Biotechnol. 2013, 23, 587–601. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Y.; Yang, L.; Guo, Y.; Fang, F.; Wang, D.; Li, R.; Jiang, M.; Kang, W.; Ma, J.; Sun, J.; et al. High-temperature cultivation of recombinant Pichia pastorisincreases endoplasmic reticulum stress and decreases production of human interleukin-10. Microb. Cell Factories 2014, 13, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, P.; Lopes, M.; Mota, M.; Belo, I. Oxygen mass transfer impact on citric acid production by Yarrowia lipolytica from crude glycerol. Biochem. Eng. J. 2016, 110, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.; Hirz, M.; Pichler, H.; Schwab, H. Protein expression in Pichia pastoris: Recent achievements and perspectives for heterologous protein production. Appl. Microbiol. Biotechnol. 2014, 98, 5301–5317. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Ly, J.; Watts, K.; Hsu, A.; Walker, A.; McLaughlin, K.; Berdichevsky, M.; Prinz, B.; Kersey, D.S.; D’Anjou, M.; et al. Optimization of a glycoengineered Pichia pastoris cultivation process for commercial antibody production. Biotechnol. Prog. 2011, 27, 1744–1750. [Google Scholar] [CrossRef]
- Çelik, E.; Çalık, P.; Oliver, S.G. Fed-batch methanol feeding strategy for recombinant protein production by Pichia pastorisin the presence of co-substrate sorbitol. Yeast 2009, 26, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Chatzifragkou, A.; Papanikolaou, S. Effect of impurities in biodiesel-derived waste glycerol on the performance and feasibility of biotechnological processes. Appl. Microbiol. Biotechnol. 2012, 95, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Venkataramanan, K.P.; Boatman, J.J.; Kurniawan, Y.; Taconi, K.A.; Bothun, G.D.; Scholz, C. Impact of impurities in biodiesel-derived crude glycerol on the fermentation by Clostridium pasteurianum ATCC 6013. Appl. Microbiol. Biotechnol. 2012, 93, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jensen, P.R.; Workman, M. Bioconversion of crude glycerol feedstocks into ethanol by Pachysolen tannophilus. Bioresour. Technol. 2012, 104, 579–586. [Google Scholar] [CrossRef]
- Vivek, N.; Sindhu, R.; Madhavan, A.; Anju, A.J.; Castro, E.; Faraco, V.; Pandey, A.; Binod, P. Recent advances in the production of value added chemicals and lipids utilizing biodiesel industry generated crude glycerol as a substrate—Metabolic aspects, challenges and possibilities: An overview. Bioresour. Technol. 2017, 239, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Duarte, S.H.; Ghiselli, G.; Maugeri, F. Influence of culture conditions on lipid production by Candida sp. LEB-M3 using glycerol from biodiesel synthesis. Biocatal. Agric. Biotechnol. 2013, 2, 339–343. [Google Scholar] [CrossRef]
- Robert, J.M.; Lattari, F.S.; Machado, A.C.; De Castro, A.M.; Almeida, R.; Torres, F.A.G.; Valero, F.; Freire, D.M.G. Production of recombinant lipase B from Candida antarctica in Pichia pastoris under control of the promoter PGK using crude glycerol from biodiesel production as carbon source. Biochem. Eng. J. 2017, 118, 123–131. [Google Scholar] [CrossRef]
- Anastácio, G.; Santos, K.; Suarez, P.; Torres, F.; De Marco, J.; Parachin, N. Utilization of glycerin byproduct derived from soybean oil biodiesel as a carbon source for heterologous protein production in Pichia pastoris. Bioresour. Technol. 2014, 152, 505–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Glycerol Type | Purity | Impurities (% w/v) |
|---|---|---|
| ≥99.5% (w/v) glycerol | G99 | ≤0.10% (w/v) water, pH 4–7 |
| 86–89% (w/v) glycerol | G86 | 11–14% (w/v) water, pH 4–7 |
| 80–85% (w/v) glycerol | G80 | 4–12% water (w/v) and 30–35% (w/v) nonglycerol organic matter, pH 9.0–9.7. |
| 65–75% (w/v) glycerol | G65 | 4–12% (w/v) water and 30–35% (w/v) nonglycerol organic matter, pH 8.3–9.0. |
| Variable Name | Variable Units | Range/Level(s) |
|---|---|---|
| Aeration | vvm | 0.50, 0.75, 1.0, 1.5 |
| Agitation | rpm | 500, 700, 1000 |
| Temperature | °C | 25, 30, 37 |
| pH | pH | 3, 4, 5, 6 |
| Model | F Test | R Square | |
|---|---|---|---|
| F Ratio | Prob > F | ||
| Linear | 3975.22 | 6.43 | 0.407 |
| Quadratic | 5163.14 | 3.95 | 0.842 |
| Glycerol Type | Reaction Rate (mg rTBL-1 L−1 h−1) i | Overall Volumetric Productivity (mg L−1 h−1) ii | Yield Biomass/Substrate (gg−1) | Yield rTBL-1/Biomass (mgg−1) | Purified rTBL-1 (mg L−1) |
|---|---|---|---|---|---|
| G99 | 1.43 ± 0.29 (a) | 1.82 ± 0.13 (a) | 1.16 ± 0.08 (a) | 1.70 ± 0.07 (a) | 274.67 ± 9.70 (a) |
| G65 | 2.04 ± 0.47 (a) | 1.65 ± 0.10 (a) | 1.08 ± 0.11 (a) | 1.65 ± 0.14 (a) | 264.95 ± 13.52 (a) |
| Glycerol Type | µmax (h−1) | qO2 (mMol g−1 X h−1) | kLa (h−1) |
|---|---|---|---|
| G99 | 0.96 | 0.99 | 115 |
| G65 | 0.93 | 0.95 | 105 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmerín-Carreño, D.; Martínez-Alarcón, D.; Dena-Beltrán, J.L.; Vega-Rojas, L.J.; Blanco-Labra, A.; Escobedo-Reyes, A.; García-Gasca, T. Optimization of a Recombinant Lectin Production in Pichia pastoris Using Crude Glycerol in a Fed-Batch System. Processes 2021, 9, 876. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9050876
Palmerín-Carreño D, Martínez-Alarcón D, Dena-Beltrán JL, Vega-Rojas LJ, Blanco-Labra A, Escobedo-Reyes A, García-Gasca T. Optimization of a Recombinant Lectin Production in Pichia pastoris Using Crude Glycerol in a Fed-Batch System. Processes. 2021; 9(5):876. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9050876
Chicago/Turabian StylePalmerín-Carreño, Dulce, Dania Martínez-Alarcón, José Luis Dena-Beltrán, Lineth Juliana Vega-Rojas, Alejandro Blanco-Labra, Antonio Escobedo-Reyes, and Teresa García-Gasca. 2021. "Optimization of a Recombinant Lectin Production in Pichia pastoris Using Crude Glycerol in a Fed-Batch System" Processes 9, no. 5: 876. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9050876