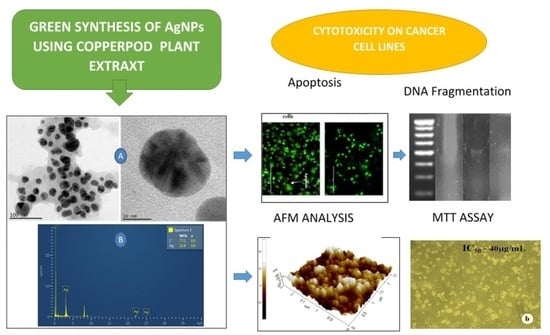
Copperpod Plant Synthesized AgNPs Enhance Cytotoxic and Apoptotic Effect in Cancer Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Extraction of Plant Materials
2.2. Synthesis and Characterization of AgNPs
2.3. Characterization of Synthesized AgNPs
2.4. In Vitro Cytotoxicity of Biogenic AgNPs
2.4.1. Cell Culture and Maintenance
2.4.2. Cell Viability Assay
2.4.3. Propidium Iodide (PI) Staining
2.4.4. Acridine Orange–Ethidium Bromide Dual Staining
2.4.5. DNA Fragmentation Analysis
3. Results and Discussions
3.1. Synthesis and Characterization of AgNPs
3.2. In Vitro Cytotoxicity Analysis of Biogenic AgNPs by MTT Assay
3.3. Propidium Iodide and Acridine Orange-Ethidium Bromide (AO-EB) Fluorescent Staining
3.4. DNA Fragmentation Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AgNPs | Silver Nanoparticles |
| UV-vis | Ultraviolet visible |
| TEM | Transmission electron microscopy |
| HRTEM | High-resolution transmission electron microscopy |
| AFM | Atomic force microscopy |
| EDX | Energy disperse X-ray |
| XRD | X-Ray Diffraction |
| HepG2 | Hepatocellular carcinoma cells |
| A549 | Lung cancer cells |
| MCF7 | Human breast cancer cells |
| ELISA | Enzyme-linked immunosorbent assay |
| DMEM | Dulbecco‘s modified eagle media |
| CO2 | Carbon dioxide |
| NCCS | National centre for cell science |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| ICP-AES | Inductively Coupled Plasma—Atomic Emission Spectrometer |
| ICP-OES | Inductively coupled plasma optical emission spectrometry |
| AO/EB | Acridine orange and ethidium bromide |
| JCPDS | Joint committee on powder diffraction standards |
| DMSO | Dimethyl sulfoxide |
| PBS | Phosphate-buffered saline |
| PI | Propidium iodide |
| fcc | Face-centred cubic |
| kb | Kilo-base |
| µg | Microgram |
| nm | Nano-meter |
| ml | Milligram |
| h | Hours |
| IC50 | The half maximal inhibitory concentration |
| G2/M | Growth 2/mitosis |
References
- Ba, T.C.; Nguyen, T.H.L.; Nguyen, T.H.A.; Tran, D.C.; Nguyen, T.T.; Dao, D.T.; Le, T.H.N.; Tram, V.S.; Ninh, T.S.; Domenico, V.D.; et al. Chemical constituents of Peltophorum pterocarpum stems. Vietnam J. Chem. 2020, 58, 569–574. [Google Scholar]
- Salata, O.V. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 2, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajipour, M.J.; Fromm, K.M.; Ali, A.A.; Aberasturi, D.J.D.; Larramendi, I.R.D.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2021, 30, 499–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.S.; Altaf, M.; Al-Lohedan, H.A. Green synthesis of biogenic silver nanoparticles using Solanum tuberosum extract and their interaction with human serum albumin: Evidence of “corona” formation through a multi-spectroscopic and molecular docking analysis. J. Photochem. Photobiol. B Biol. 2017, 173, 108–119. [Google Scholar] [CrossRef]
- Moritz, M.; Geszke-Moritz, M. The newest achievements in synthesis, immobilization and practical applications of antibacterial nanoparticles. Chem. Eng. J. 2013, 228, 596–613. [Google Scholar] [CrossRef]
- Ramos, A.P.; Cruz, M.A.E.; Tovani, C.B.; Ciancaglini, P. Biomedical applications of nanotechnology. Biophys. Rev. 2017, 9, 79–89. [Google Scholar] [CrossRef]
- Siva, S.S.; Huizhen, L.; Zhijun, Z.; Minaxi, S.; Zeba, U.; Tianyu, H.; Vasudeva, R.N.; Xin, W.; Vijai, K.G. Recent advances in essential oils-based metal nanoparticles: A review on recent developments and biopharmaceutical applications. J. Mol. Liquids 2021, 333, 115951. [Google Scholar]
- Paiva-Santos, A.C.; Herdade, A.M.; Guerra, C.; Peixoto, D.; Pereira-Silva, M.; Zeinali, M.; Mascarenhas-Melo, F.; Paranhos, A.; Veiga, F. Plant-mediated green synthesis of metal-based nanoparticles for dermopharmaceutical and cosmetic applications. Int. J. Pharm. 2021, 597, 120311. [Google Scholar] [CrossRef]
- Ueda, M.; Yokota, T.; Honda, M.; Lim, P.N.; Osaka, N.; Makita, M.; Nishikawa, Y.; Kasuga, T.; Aizawa, M. Regulating size of silver nanoparticles on calcium carbonate via ultrasonic spray for effective antibacterial efficacy and sustained release. Mater. Sci. Eng. C. 2021, 125, 112083. [Google Scholar] [CrossRef]
- Taiba, N.; Tayyiba, D. The role of some important metal oxide nanoparticles for wastewater and antibacterial applications: A review. Environ. Chem. Ecotoxicol. 2021, 3, 59–75. [Google Scholar]
- Saravanan, A.; Senthil Kumar, B.; Karishma, S.; Dai-Viet, N.V.; Jeevanantham, S.; Yaashikaa, P.R.; George, C.S. A review on biosynthesis of metal nanoparticles and its environmental applications. Chemosphere 2021, 264, 128580. [Google Scholar] [CrossRef]
- Behzad, F.; Naghib, S.M.; Kouhbanani, M.A.J.; Tabatabaei, S.N.; Zare, Y.; Rhee, K.Y. An overview of the plant-mediated green synthesis of noble metal nanoparticles for antibacterial applications. J. Ind. Eng. Chem. 2021, 94, 92–104. [Google Scholar] [CrossRef]
- Mittal, A.K.; Tripathy, D.; Choudhary, A.; Ali, P.K.; Chatterjee, A.; Singh, I.P.; Banerjee, U.C. Bio-synthesis of silver nanoparticles using Potentilla fulgens Wall. ex Hook. and its therapeutic evaluation as anticancer and antimicrobial agent. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 53, 120–127. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—An updated report. Saudi. Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef]
- Nakkala, J.R.; Mata, R.; Gupta, A.K.; Sadras, S.R. Biological activities of green silver nanoparticles synthesized with Acorous calamus rhizome extract. Eur. J. Med. Chem. 2014, 85, 784–794. [Google Scholar] [CrossRef]
- Ahamed, M.; AlSalhi, M.S.; Siddiqui, M.K.J. Silver nanoparticle applications and human health. Clin. Chim. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176. [Google Scholar] [CrossRef]
- Milad, A.; Ali, Z.; Farid, H.; Ebrahim, R.M.; Fardin, H.; Maliheh, E.; Kiavash, H.; Reza, M.; Masoud, N. Curcumin in cancer therapy: A novel adjunct for combination chemotherapy with paclitaxel and alleviation of its adverse effects. Life Sci. 2020, 256, 117984. [Google Scholar]
- Jyoti, S.; Suaib, L.; Abha, M. Emerging role of phytochemicals in targeting predictive, prognostic, and diagnostic biomarkers of lung cancer. Food Chem. Toxicol. 2020, 144, 111592. [Google Scholar]
- Yonglu, L.; Xiaodong, Z.; Qiang, C. Bio-based nanomaterials for cancer therapy. Nano Today 2021, 38, 101134. [Google Scholar]
- Mehran, A.; Rajender, S.V. Phytosynthesis and modification of metal and metal oxide nanoparticles/nanocomposites for antibacterial and anticancer activities: Recent advances. Sust. Chem. Pharm. 2021, 21, 100412. [Google Scholar]
- Quan, L.; Yanhong, D.; Jianye, F.; Meng, Q.; Zhe, S.; Dickson, A.; Jianlong, K.; Zhongjian, X.; Taojian, F.; Shiyun, B.; et al. Nano-immunotherapy: Unique mechanisms of nanomaterials in synergizing cancer immunotherapy. Nano Today 2021, 36, 101023. [Google Scholar]
- Mohammad, R.S.; Sheyda, R.; Ivan, M.K.; Mostafa, A.; Willis, C.A.M.; Mareike, M.; Mohammad, H.G.; Mohammad, R. Targeting non-apoptotic cell death in cancer treatment by nanomaterials: Recent advances and future outlook. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102243. [Google Scholar]
- Dos Santos, C.A.; Seckler, M.M.; Ingle, A.P.; Gupta, I.; Gladiero, S.; Gladiero, M.; Gade, A.; Rai, M. Silver nanoparticles: Therapeutical uses, toxicity, and safety issues. J. Pharm. Sci. 2014, 103, 1931–1944. [Google Scholar] [CrossRef]
- Rizzello, L.; Pompa, P.P. Nanosilver-based antibacterial drugs and devices: Mechanisms, methodological drawbacks, and guidelines. Chem. Soc. Rev. 2009, 43, 1501–1518. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver nanoparticles: Green synthesis and their antimicrobial activities. Adv. Colloid. Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef]
- Mostafa, F.A.H.; Gamal, A.G.; Sedky, H.A.H. A review of green methods for phyto-fabrication of hematite (α-Fe2O3) nanoparticles and their characterization, properties, and applications. Heliyon 2021, 7, e05806. [Google Scholar]
- Xu, Z.P.; Zeng, Q.H.; Lu, G.Q.; Yu, A.B. Inorganic nanoparticles as carriers for efficient cellular delivery. Chem. Eng. Sci. 2006, 61, 1027–1040. [Google Scholar] [CrossRef]
- Jing, H.; Yunlei, X. When nano meets plants: A review on the interplay between nanoparticles and plants. Nano Today 2021, 38, 101143. [Google Scholar]
- Muhammad, S.R.R.; Riaz, R.; Hafiza, M.M.; Yongai, X.; Xun, S.; Nazim, H.; Qinchang, Z.; Zhendan, H. Gut microbiota targeted nanomedicine for cancer therapy: Challenges and future considerations. Trends Food Sci. Technol. 2021, 107, 240–251. [Google Scholar]
- Arul, K.M.; Balashanmugam, P.; Javee, A.; Rajenderan, M.; Devasena, T. Facile green synthesis and characterization of Gloriosa superba L. tuber extract-capped silver nanoparticles (GST-AgNPs) and its potential antibacterial and anticancer activities against A549 human cancer cells. Environ. Nanotechnol. Monitoring Manag. 2021, 15, 10046. [Google Scholar]
- Violeta, M.L.; Heriberto, E.G.; Lucía, Z.F.L.; Erika, L.S.B.; Alfredo, N.R.; Rubén, D.C.N.; Gabriel, A.N.; Ignacio, A.R. Study of the effect of the different parts of Morinda citrifolia L. (noni) on the green synthesis of silver nanoparticles and their antibacterial activity. Appl. Surf. Sci. 2021, 537, 147855. [Google Scholar]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanoparticle Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Shohreh, F.; Fatemeh, A.; Mansour, G. Synthesis and therapeutic potential of silver nanomaterials derived from plant extracts. Ecotoxicol. Environ. Safety 2019, 168, 260–278. [Google Scholar]
- Balashanmugam, P.; Prabhu, D.; Devasena, T.; Hak, J.S.; Kim, K.W.; Jung, Y.S.; Song, H.J.; Kim, H.J.; Kumaran, R.S. Facile synthesis of silver nanoparticles using Asian spider flower and its in vitro cytotoxic activity against human breast carcinoma cells. Processes 2020, 8, 430. [Google Scholar]
- Harish, C.; Pragati, K.; Elza, B.; Saurabh, Y. Medicinal plants: Treasure trove for green synthesis of metallic nanoparticles and their biomedical applications. Biocatal. Agricult. Biotechnol. 2020, 24, 101518. [Google Scholar]
- Kathiravan, G.; Yamini, K.R.; Rajagopal, K.; Anandan, S.; Kim, K.W.; Jung, Y.S.; Song, H.J.; Kim, H.J.; Kumaran, R.S. Phytogenic synthesis of nano silver from Madagascar Periwinkle extracts and their angiogenic activities in Zebrafish embryos (ZFE). Nanosci. Nanotechnol. Lett. 2020, 12, 79–87. [Google Scholar] [CrossRef]
- Li, Y.C.; Kuo, P.C.; Yang, M.L.; Chen, T.Y.; Hwang, T.L.; Chiang, C.C.; Thang, T.D.; Tuan, N.N.; Tzen, J.T.C. Chemical constituents of the leaves of Peltophorum pterocarpum and their bioactivity. Molecules 2019, 24, 240. [Google Scholar] [CrossRef] [Green Version]
- Sukumaran, S.; Kiruba, S.; Mahesh, M.; Nisha, S.R.; Miller, P.Z.; Ben, C.P.; Jeeva, S. Phytochemical constituents and antibacterial efficacy of the flowers of Peltophorum pterocarpum (DC.) Baker ex Heyne. Asian Pacific J. Trop. Med. 2011, 4, 735–738. [Google Scholar] [CrossRef] [Green Version]
- Annamalai, P.; Balashanmugam, P.; Kalaichelvan, P.T. In vitro wound healing and antimicrobial property of cotton fabrics coated optimized silver nanoparticles synthesized using Peltophorum pterocarpum leaf extracts. Asian J. Pharm. Clin. Res. 2019, 12, 216–222. [Google Scholar] [CrossRef]
- Annamalai, P.; Balashanmugam, P.; Kalaichelvan, P.T. Biogenic synthesis silver nanoparticles using Peltophorum pterocarpum leaf extracts and its antimicrobial efficacy against selective pathogens. Int. J. App. Pharm. 2018, 10, 112–118. [Google Scholar] [CrossRef] [Green Version]
- Balashanmugam, P.; Kalaichelvan, P.T. Biosynthesis characterization of silver nanoparticles using Cassia roxburghii DC. aqueous extract and coated on cotton cloth for effective antibacterial activity. Int. J. Nanomed. 2015, 10, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, K.; Panghal, M.; Kadyan, S.; Chaudhary, U.; Yadav, J.P. Green silver nanoparticles of Phyllanthus amarus: As an antibacterial agent against multi drug resistant clinical isolates of Pseudomonas aeruginosa. J. Nanobiotechnol. 2014, 12, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balashanmugam, P.; Balakumaran, M.D.; Murugan, R.; Dhanapal, K.; Kalaichelvan, P.T. Phytogenic synthesis of silver nanoparticles, optimization and evaluation of in vitro antifungal activity against human and plant pathogens. Mibiol. Res. 2016, 192, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Almutary, A.; Sanderson, B.J.S. The MTT and crystal violet assays: Potential confounders in nanoparticle toxicity testing. Int. J. Toxicol. 2016, 35, 454–462. [Google Scholar] [CrossRef]
- Devanesan, S.; Alsalhi, M.S.; Vishnubalaji, R.; Alfuraydi, A.A.; Alajez, N.M.; Alfayez, M.; Murugan, K.; Sayed, S.R.M.; Nicoletti, M.; Benelli, G. Rapid biological synthesis of silver nanoparticles using plant seed extracts and their cytotoxicity on colorectal cancer cell lines. J. Clust. Sci. 2017, 28, 595–605. [Google Scholar] [CrossRef]
- Dey, A.; Mukhopadhyay, A.K.; Gangadharan, S.; Sinha, M.K.; Basu, D.; Bandyopadhyay, N.R. Nanoindentation study of microplasma sprayed hydroxyapatite coating. Ceram. Int. 2009, 35, 2295–2304. [Google Scholar] [CrossRef]
- Paulkumar, K.; Rajeshkumar, S.; Gnanajobitha, G.; Vanaja, M.; Malarkodi, C.; Annadurai, G. Biosynthesis of silver chloride nanoparticles using Bacillus subtilis MTCC 3053 and assessment of its antifungal activity. ISRN Nanomater. 2013, 317963. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, N.; Muddapur, U. Biosynthesis of metal nanoparticles: A review. J. Nanotechnol. 2014, 510246. [Google Scholar] [CrossRef] [Green Version]
- Mohamad, N.A.N.; Arham, N.A.; Jai, J.; Hadi, A. Plant extract as reducing agent in synthesis of metallic nanoparticles: A review. Nanosci. Nanotechnol. Nanoeng. 2014, 832, 350–355. [Google Scholar] [CrossRef]
- Carmona, E.R.; Benito, N.; Plaza, T.; Recio-Sánchez, G. Green synthesis of silver nanoparticles by using leaf extracts from the endemic Buddleja globosa Hope. Green Chem. Lett. Rev. 2017, 10, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, P.; Satapathy, M.; Mukhopahayay, A.; Das, P. Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: Synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour. Bioprocess. 2014, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Moteriya, P.; Chanda, S. Biosynthesis of silver nanoparticles formation from Caesalpinia pulcherrima stem metabolites and their broad spectrum biological activities. J. Genet. Eng. Biotechnol. 2018, 16, 105–113. [Google Scholar] [CrossRef]
- Rónavári, A.; Kovács, D.; Igaz, N.; Vagvolgyi, C.; Boros, I.M.; Konya, Z.; Pfeiffer, I.; Kiricsi, M. Biological activity of green-synthesized silver nanoparticles depends on the applied natural extracts: A comprehensive study. Int. J. Nanomed. 2017, 12, 871–883. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Chowdhury, D.; Kotcherlakota, R.; Patra, S.; Vinothkumar, B.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics 2014, 4, 316–335. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Sushma, V.; Patra, S.; Barui, A.K.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Green chemistry approach for the synthesis and stabilization of biocompatible gold nanoparticles and their potential applications in cancer therapy. Nanotechnology 2012, 23, 455103. [Google Scholar] [CrossRef]
- Mukherjee, S.; Vinothkumar, B.; Prashanthi, S.; Bangal, P.R.; Sreedhar, B.; Patra, C.R. Potential therapeutic and diagnostic applications of one-step in situ biosynthesized gold nanoconjugates (2-in-1 system) in cancer treatment. RSC Adv. 2013, 3, 2318–2329. [Google Scholar] [CrossRef]
- Jadhav, K.; Dhamecha, D.; Bhattacharya, D.; Patil, M. Green and ecofriendly synthesis of silver nanoparticles: Characterization, biocompatibility studies and gel formulation for treatment of infections in burns. J. Photochem. Photobiol. B. Biol. 2016, 155, 109–115. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Ji, B.J.; Harper, S.L.; Yun, S.I. Plant extract-mediated biogenic synthesis of silver, manganese dioxide, silver-doped manganese dioxide nanoparticles and their antibacterial activity against food- and water-borne pathogens. Bioprocess. Biosyst. Eng. 2016, 39, 759–772. [Google Scholar] [CrossRef]
- Mishra, P.M.; Sahoo, S.K.; Naik, G.K.; Parida, K. Biomimetic synthesis, characterization and mechanism of formation of stable silver nanoparticles using Averrhoa carambola L. leaf extract. Mater. Lett. 2015, 160, 566–571. [Google Scholar] [CrossRef]
- Khatami, M.; Nejad, M.S.; Almani, P.G.N.; Salari, S. Plant-mediated green synthesis of silver nanoparticles using Trifolium resupinatum seed exudate and their antifungal efficacy on Neofusicoccum parvum and Rhizoctonia solani. IET Nanobiotechnol. 2016, 10, 237–243. [Google Scholar] [CrossRef]
- Nasar, M.Q.; Khalil, A.T.; Ali, M.; Shah, M.; Ayaz, M.; Shinwari, Z.K. Phytochemical analysis, Ephedra procera C. A. Mey. mediated green synthesis of silver nanoparticles, their cytotoxic and antimicrobial potentials. Medicina 2019, 55, 369. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.; Anwar, F.; Janjua, M.R.S.A.; Iqbal, M.A.; Rashid, U. Green synthesis of silver nanoparticles through reduction with Solanum xanthocarpum L. berry extract: Characterization, antimicrobial and urease inhibitory activities against Helicobacter pylori. Int. J. Mol. Sci. 2012, 13, 9923–9941. [Google Scholar] [CrossRef]
- Khalil, M.M.H.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab. J. Chem. 2014, 7, 1131–1139. [Google Scholar] [CrossRef] [Green Version]
- Daphedar, A.; Taranath, T.C. Characterization and cytotoxic effect of biogenic silver nanoparticles on mitotic chromosomes of Drimia polyantha (Blatt. & McCann) Stearn. Toxicol. Rep. 2018, 5, 910–918. [Google Scholar]
- Ajitha, B.; Reddy, Y.A.K.; Reddy, P.S. Green synthesis and characterization of silver nanoparticles using Lantana camara leaf extract. Mater. Sci. Eng. C. 2015, 49, 373–381. [Google Scholar] [CrossRef]
- Yasir, M.; Singh, J.; Tripathi, M.K.; Singh, P.; Shrivastava, R. Green synthesis of silver nanoparticles using leaf extract of common arrowhead houseplant and its anticandidal activity. Pharmacogn. Mag. 2017, 13, S840–S844. [Google Scholar]
- Abbasi, B.H.; Nazir, M.; Muhammad, W.; Hashmi, S.S.; Abbasi, R.; Rahman, L.; Hano, C. A comparative evaluation of the antiproliferative activity against HepG2 liver carcinoma cells of plant-derived silver nanoparticles from basil extracts with contrasting anthocyanin contents. Biomolecules 2019, 9, 320. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Tiwari, R.; Singh, V.K.; Singh, P.; Khadim, S.R.; Singh, U.; Laxmi; Srivastava, V.; Hasan, S.H.; Asthana, R.K. Green synthesis of gold nanoparticles from Dunaliella salina, its characterization and in vitro anticancer activity on breast cancer cell line. J. Drug Deliv. Sci. Technol. 2019, 51, 164–176. [Google Scholar] [CrossRef]
- Moldovan, B.; Sincari, V.; Perde-Schrepler, M.; David, L. Biosynthesis of Silver Nanoparticles Using Ligustrum Ovalifolium Fruits and Their Cytotoxic Effects. Nanomaterials 2018, 8, 627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asha, R.P.V.; Kah, M.G.L.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar]
- Venugopal, K.; Rather, H.A.; Rajagopal, K.; Shanthi, M.P.; Sheriff, K.; Illiyas, M.; Rather, R.A.; Manikandan, E.; Uvarajan, S.; Bhaskar, M.; et al. Synthesis of silver nanoparticles (Ag NPs) for anticancer activities (MCF 7 breast and A549 lung cell lines) of the crude extract of Syzygium aromaticum. J. Photochem. Photobiol. B Biol. 2017, 167, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Satpathy, S.; Patra, A.; Ahirwar, B.; Hussain, M.D. Antioxidant and anticancer activities of green synthesized silver nanoparticles using aqueous extract of tubers of Pueraria tuberosa. Artif. Cells Nanomed. Biotechnol. 2018, 46, S71–S85. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Zhou, L.; Rajoka, M.S.R.; Yuan, L.; Jiang, C.; Shao, D.; Zhu, J.; Shi, J.; Huang, Q.; Yang, H.; et al. Fungal silver nanoparticles: Synthesis, application and challenges. Crit. Rev. Biotechnol. 2018, 38, 817–835. [Google Scholar] [CrossRef]
- Chokkalingam, M.; Singh, P.; Huo, Y.; Soshnikova, V.; Ahn, S.; Kang, J.; Mathiyalagan, R.; Kim, Y.J.; Yang, D.C. Facile synthesis of Au and Ag nanoparticles using fruit extract of Lycium chinense and their anticancer activity. J. Drug Deliv. Sci. Technol. 2019, 4, 308–315. [Google Scholar] [CrossRef]
- Alsalhi, M.S.; Devanesan, S.; Alfuraydi, A.A.; Vshnubalaji, R.; Munusamy, M.A.; Murugan, K.; Nivoletti, M.; Benelli, G. Green synthesis of silver nanoparticles using Pimpinella anisum seeds: Antimicrobial activity and cytotoxicity on human neonatal skin stromal cells and colon cancer cells. Int. J. Nanomed. 2016, 11, 4439–4449. [Google Scholar] [CrossRef] [Green Version]
- Mata, R.; Nakkala, J.R.; Sadras, S.R. Biogenic silver nanoparticles from Abutilon indicum: Their antioxidant, antibacterial and cytotoxic effects in vitro. Coll. Surf. B. Biointerfaces 2015, 128, 276–286. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Chelliah, R.; Shanmugam, S.; Varukattu, N.B.; Oh, D.H.; Kathiresan, K.; Wang, M.H. Green synthesis and characterization of biologically active nanosilver from seed extract of Gardenia jasminoides Ellis. J. Photochem. Photobiol. B. Biol. 2018, 185, 126–135. [Google Scholar] [CrossRef]
- Balashanmugam, P.; Durai, P.; Balakumaran, M.D.; Kalaichelvan, P.T. Phytosynthesized gold nanoparticles from C. roxburghii DC. leaf and their toxic effects on normal and cancer cell lines. J. Photochem. Photobiol. B Biol. 2016, 165, 163–173. [Google Scholar] [CrossRef]
- Hanna, D.H.; Saad, G.R. Nanocurcumin: Preparation, characterization and cytotoxic effects towards human laryngeal cancer cells. RSC Adv. 2020, 10, 20724–20737. [Google Scholar] [CrossRef]
- Sangour, M.H.; Ali, I.M.; Atwan, Z.W.; Ali, A.A.A.L.A.A. Effect of Ag nanoparticles on viability of MCF-7 and Vero cell lines and gene expression of apoptotic genes. Egypt. J. Med. Hum. Genet. 2021, 22, 9. [Google Scholar] [CrossRef]
- Fard, N.N.; Noorbazargan, H.; Mirzaie, A.; Hedayati Ch, M.; Moghimiyan, Z.; Rahimi, A. Biogenic synthesis of AgNPs using Artemisia oliveriana extract and their biological activities for an effective treatment of lung cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, S1047–S1058. [Google Scholar] [CrossRef] [Green Version]
- Krishnaraj, C.; Muthukumaran, P.; Ramachandran, R.; Balakumaran, M.D.; Kalaichelvan, P.T. Acalypha indica Linn: Biogenic synthesis of silver and gold nanoparticles and their cytotoxic effects against MDA-MB-231, human breast cancer cells. Biotechnol. Rep. 2014, 4, 42–49. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pannerselvam, B.; Thiyagarajan, D.; Pazhani, A.; Thangavelu, K.P.; Kim, H.J.; Rangarajulu, S.K. Copperpod Plant Synthesized AgNPs Enhance Cytotoxic and Apoptotic Effect in Cancer Cell Lines. Processes 2021, 9, 888. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9050888
Pannerselvam B, Thiyagarajan D, Pazhani A, Thangavelu KP, Kim HJ, Rangarajulu SK. Copperpod Plant Synthesized AgNPs Enhance Cytotoxic and Apoptotic Effect in Cancer Cell Lines. Processes. 2021; 9(5):888. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9050888
Chicago/Turabian StylePannerselvam, Balashanmugam, Devasena Thiyagarajan, Annamalai Pazhani, Kalaichelvan Pudupalayam Thangavelu, Hyung Joo Kim, and Senthil Kumaran Rangarajulu. 2021. "Copperpod Plant Synthesized AgNPs Enhance Cytotoxic and Apoptotic Effect in Cancer Cell Lines" Processes 9, no. 5: 888. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9050888