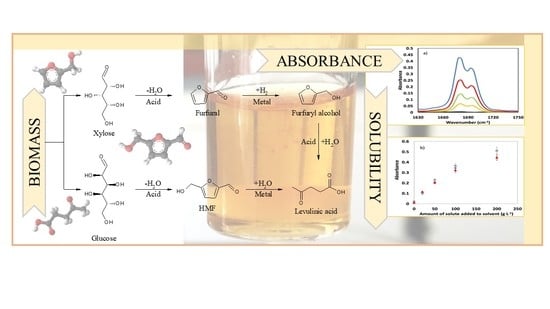
Solubility Temperature Dependence of Bio-Based Levulinic Acid, Furfural, and Hydroxymethylfurfural in Water, Nonpolar, Polar Aprotic and Protic Solvents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiment Procedure
2.3. Analytical Method
3. Results
3.1. FTIR Spectrum of Furfural, Levulinic Acid, and Hydroxymethylfurfural
3.2. Concentration Dependent Solubility
3.3. Signal Dependence at Different Temperatures
3.4. Solubility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gautam, P.; Upadhyay, S.N.; Dubey, S.K. Bio-methanol as a renewable fuel from waste biomass: Current trends and future perspective. Fuel 2020, 273, 117783. [Google Scholar] [CrossRef]
- Wei, H.; Liu, W.; Chen, X.; Yang, Q.; Li, J.; Chen, H. Renewable bio-jet fuel production for aviation: A review. Fuel 2019, 254, 115599. [Google Scholar] [CrossRef]
- Jeong, G.-T.; Kim, S.-K. Thermochemical conversion of defatted microalgae Scenedesmus obliquus into levulinic and formic acids. Fuel 2021, 283, 118907. [Google Scholar] [CrossRef]
- Madduluri, V.R.; Mandari, K.K.; Velpula, V.; Varkolu, M.; Kamaraju, S.R.R.; Kang, M. Rice husk-derived carbon-silica supported Ni catalysts for selective hydrogenation of biomass-derived furfural and levulinic acid. Fuel 2020, 261, 116339. [Google Scholar] [CrossRef]
- Remón, J.; Casales, M.; Gracia, J.; Callén, M.S.; Pinilla, J.L.; Suelves, I. Sustainable production of liquid biofuels and value-added platform chemicals by hydrodeoxygenation of lignocellulosic bio-oil over a carbon–neutral Mo2C/CNF catalyst. Chem. Eng. J. 2021, 405, 126705. [Google Scholar] [CrossRef]
- Ge, S.; Yek, P.N.Y.; Cheng, Y.W.; Xia, C.; Wan Mahari, W.A.; Liew, R.K.; Peng, W.; Yuan, T.-Q.; Tabatabaei, M.; Aghbashlo, M.; et al. Progress in microwave pyrolysis conversion of agricultural waste to value-added biofuels: A batch to continuous approach. Renew. Sustain. Energy Rev. 2021, 135, 110148. [Google Scholar] [CrossRef]
- Tomasek, S.; Lónyi, F.; Valyon, J.; Hancsók, J. Fuel purpose hydrocracking of biomass based Fischer-Tropsch paraffin mixtures on bifunctional catalysts. Energy Convers. Manag. 2020, 213, 112775. [Google Scholar] [CrossRef]
- Riisager, A.; Jensen, J.S.; Ståhlberg, T.J.B.; Woodley, J. Purification of 5-Hydroxymethylfurfural (HMF) by Crystallization 2013. WO 2013/024162 A1, 21 February 2013. [Google Scholar]
- Li, X.; Jia, P.; Wang, T. Furfural: A Promising Platform Compound for Sustainable Production of C4 and C5 Chemicals. ACS Catal. 2016, 6, 7621–7640. [Google Scholar] [CrossRef]
- Dalessandro, E.V.; Pliego, J.R. Fast screening of solvents for simultaneous extraction of furfural, 5-hydroxymethylfurfural and levulinic acid from aqueous solution using SMD solvation free energies. J. Braz. Chem. Soc. 2018. [Google Scholar] [CrossRef]
- Morone, A.; Apte, M.; Pandey, R.A. Levulinic acid production from renewable waste resources: Bottlenecks, potential remedies, advancements and applications. Renew. Sustain. Energy Rev. 2015, 51, 548–565. [Google Scholar] [CrossRef]
- Climent, M.J.; Corma, A.; Iborra, S. Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels. Green Chem. 2014, 16, 516–547. [Google Scholar] [CrossRef] [Green Version]
- Werpy, T.A.; Holladay, J.; White, J. Top Value Added Chemicals from Biomass: I. Results of Screening for Potential Candidates from Sugars and Synthesis Gas; Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2004. [Google Scholar]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Li, J.; Jia, C.; Shao, J.; Yang, Q.; Chen, Y.; Yang, H.; Wang, X.; Chen, H. Preparation of furfural by catalytic pyrolysis of cellulose based on nano Na/Fe-solid acid. Fuel 2019, 258, 116089. [Google Scholar] [CrossRef]
- Elsayed, I.; Mashaly, M.; Eltaweel, F.; Jackson, M.A.; Hassan, E.B. Dehydration of glucose to 5-hydroxymethylfurfural by a core-shell Fe3O4@SiO2-SO3H magnetic nanoparticle catalyst. Fuel 2018, 221, 407–416. [Google Scholar] [CrossRef]
- Le Guillou, C.; Bernard, S.; De la Pena, F.; Le Brech, Y. XANES-Based Quantification of Carbon Functional Group Concentrations. Anal. Chem. 2018, 90, 8379–8386. [Google Scholar] [CrossRef]
- Terrell, E.; Carré, V.; Dufour, A.; Aubriet, F.; Le Brech, Y.; Garcia-Pérez, M. Contributions to Lignomics: Stochastic Generation of Oligomeric Lignin Structures for Interpretation of MALDI–FT-ICR-MS Results. ChemSusChem 2020, 13, 4428–4445. [Google Scholar] [CrossRef]
- Rose, H.B.; Greinert, T.; Held, C.; Sadowski, G.; Bommarius, A.S. Mutual Influence of Furfural and Furancarboxylic Acids on Their Solubility in Aqueous Solutions: Experiments and Perturbed-Chain Statistical Associating Fluid Theory (PC-SAFT) Predictions. J. Chem. Eng. Data 2018, 63, 1460–1470. [Google Scholar] [CrossRef]
- Chatterjee, A.; HU, X.; Lam, F.L.-Y. Modified coal fly ash waste as an efficient heterogeneous catalyst for dehydration of xylose to furfural in biphasic medium. Fuel 2019, 239, 726–736. [Google Scholar] [CrossRef]
- Guillory, J.K. Handbook of Aqueous Solubility Data By Samuel H. Yalkowsky and Yan He. J. Med. Chem. 2003, 46, 4213. [Google Scholar] [CrossRef]
- Vauthey, S.; Leser, M.E.; Garti, N.; Watzke, H.J. Solubilization of Hydrophilic Compounds in Copolymer Aggregates. J. Colloid Interface Sci. 2000, 225, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Hertel, M.O.; Scheuren, H.; Sommer, K. Solubilities of Hexanal, Benzaldehyde, 2-Furfural, 2-Phenylethanol, Phenylethanal, and γ-Nonalactone in Water at Temperatures between (50 and 100) °C. J. Chem. Eng. Data 2007, 52, 2143–2145. [Google Scholar] [CrossRef]
- Anthonia, E.E.; Philip, H.S. An overview of the applications of furfural and its derivatives. Int. J. Adv. Chem. 2015, 3, 42. [Google Scholar] [CrossRef]
- Stark, A.; Ondruschka, B.; Zaitsau, D.H.; Verevkin, S.P. Biomass-Derived Platform Chemicals: Thermodynamic Studies on the Extraction of 5-Hydroxymethylfurfural from Ionic Liquids. J. Chem. Eng. Data 2012, 57, 2985–2991. [Google Scholar] [CrossRef]
- Gajula, S.; Inthumathi, K.; Arumugam, S.R.; Srinivasan, K. Strategic Designing on Selection of Solvent Systems for Conversion of Biomass Sugars to Furan Derivatives and Their Separation. ACS Sustain. Chem. Eng. 2017, 5, 5373–5381. [Google Scholar] [CrossRef]
- Payne, S.M.; Kerton, F.M. Solubility of bio-sourced feedstocks in ‘green’ solvents. Green Chem. 2010, 12, 1648–1653. [Google Scholar] [CrossRef] [Green Version]
- Apelblat, A.; Manzurola, E. Solubility of suberic, azelaic, levulinic, glycolic, and diglycolic acids in water from 278.25 K to 361.35 K. J. Chem. Thermodyn. 1990, 22, 289–292. [Google Scholar] [CrossRef]
- Selva, M.; Gottardo, M.; Perosa, A. Upgrade of Biomass-Derived Levulinic Acid via Ru/C-Catalyzed Hydrogenation to γ-Valerolactone in Aqueous–Organic–Ionic Liquids Multiphase Systems. ACS Sustain. Chem. Eng. 2013, 1, 180–189. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W.; Yip, A.C.K.; Hunt, A.J.; Sherwood, J.; Shang, J.; Song, H.; Ok, Y.S.; Poon, C.S. Propylene carbonate and γ-valerolactone as green solvents enhance Sn(IV)-catalysed hydroxymethylfurfural (HMF) production from bread waste. Green Chem. 2018, 20, 2064–2074. [Google Scholar] [CrossRef]
- Dutta, S.; Yu, I.K.M.; Tsang, D.C.W.; Fan, J.; Clark, J.H.; Jiang, Z.; Su, Z.; Hu, C.; Poon, C.S. Efficient Depolymerization of Cellulosic Paper Towel Waste Using Organic Carbonate Solvents. ACS Sustain. Chem. Eng. 2020, 8, 13100–13110. [Google Scholar] [CrossRef]
- Hodgkinson, M.C.; Sagatys, D.S.; Mackey, A.P.; Smith, G. Quantitative FTIR Method for Determination of Mineral Oil Deposited on Plant Leaves. J. Agric. Food Chem. 1995, 43, 2794–2795. [Google Scholar] [CrossRef]
- Rytwo, G.; Zakai, R.; Wicklein, B. The Use of ATR-FTIR Spectroscopy for Quantification of Adsorbed Compounds. J. Spectrosc. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- George, K.M.; Ruthenburg, T.C.; Smith, J.; Yu, L.; Zhang, Q.; Anastasio, C.; Dillner, A.M. FT-IR quantification of the carbonyl functional group in aqueous-phase secondary organic aerosol from phenols. Atmos. Environ. 2015, 100, 230–237. [Google Scholar] [CrossRef]
- Königsberger, E. Editorial: Guidelines for the Measurement of Solid–Liquid Solubility Data at Atmospheric Pressure. J. Chem. Eng. Data 2019, 64, 381–385. [Google Scholar] [CrossRef] [Green Version]
- Ballester, L.; Carrió, C.; Bertran, J.F. Fermi resonance in rotational isomers of furfural. Spectrochim. Acta Part A Mol. Spectrosc. 1972, 28, 2103–2112. [Google Scholar] [CrossRef]
- Ong, H.K.; Sashikala, M. Identification of furfural synthesized from pentosan in rice husk. J. Trop. Agric. food Sci. 2007, 35, 305–312. [Google Scholar]
- Zakzeski, J.; Grisel, R.J.H.; Smit, A.T.; Weckhuysen, B.M. Solid acid-catalyzed cellulose hydrolysis monitored by in situ Atr-Ir spectroscopy. ChemSusChem 2012, 5, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Auger, E.; Coquelet, C.; Valtz, A.; Nala, M.; Naidoo, P.; Ramjugernath, D. Equilibrium data and GC-PC SAFT predictions for furanic extraction. Fluid Phase Equilib. 2016, 430, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Chen, E.Y.-X. Integrated catalytic process for biomass conversion and upgrading to C 12 furoin and alkane fuel. ACS Catal. 2014, 4, 1302–1310. [Google Scholar] [CrossRef]
- Urbanus, J.H.; Verodes, D.; Engel, C.A.R. Crystallization of Furanic Compounds. WO 2016/068712 A1, 6 May 2016. [Google Scholar]
- Rocha, D.C.; de Sampaio, H.A.C.; de Lima, J.W.O.; Carioca, A.A.F.; Lima, G.P.; de Oliveira, T.R.; Vergara, C.M.A.C.; Pinheiro, D.C.S.N.; Herculano, J.R.L.; de Ellery, T.H.P. Asociación de la infección por Helicobacter pylori con el estado nutricional y la ingesta de alimentos. Nutr. Hosp. 2015, 32, 905–912. [Google Scholar] [CrossRef]
- Domanska, U. Chapter 8. Solubility of Organic Solids for Industry. In Developments and Applications in Solubility; Royal Society of Chemistry: Cambridge, UK, 2007; pp. 94–130. ISBN 978-1-84755-768-1. [Google Scholar]
- Larrañaga, M.D.; Lewis, R.J.; Lewis, R.A. Hawley’s Condensed Chemical Dictionary, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; Volume 46, ISBN 9781119312468. [Google Scholar]
- Milne, G.W.A.; Lide, D.R. Handbook of Data on Common Organic Compounds; CRC press: Boca Raton, FL, USA, 1995. [Google Scholar]
- O’Neil, M.J.; Heckelman, P.E.; Dobbelaar, P.H.; Britain, R.S.; Roman, K.J.; Kenny, C.M.; Karaffa, L.S. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals; Merck Index; Royal Society of Chemistry: Cambridge, UK, 2013; ISBN 9781849736701. [Google Scholar]



| Name | Purity | Source | CAS Number | Tm [°C] |
|---|---|---|---|---|
| Levulinic acid | 98% | Sigma-Aldrich | 123-76-2 | 30–33 |
| Furfural | 99% | Sigma-Aldrich | 98-01-1 | −36 |
| Hydroxymethylfurfural | 67-47-0 | 28–33 | ||
| n-Hexane | 98.5% | Merck | 110-54-3 | −94 |
| Cyclohexane | 99.5% | Elixir Zorka Šabac | 110-82-7 | 6.5 |
| Benzene | 99.0% | Honeywell | 71-43-2 | 5.5 |
| Toluene | 99.7% | Honeywell | 108-88-3 | −95.0 |
| 1,4-dioxane | 99% | Honeywell | 123-91-1 | 12 |
| Diethyl ether | 99.5% | Merck | 60-29-7 | −116.3 |
| Dichloromethane | 99.5% | Merck | 75-09-2 | −95 |
| THF | 99% | Sigma-Aldrich | 96-47-9 | −108 |
| Ethyl acetate (ETOAc) | 99.9% | Honeywell | 141-78-6 | −84 |
| Acetone | 99.5% | Honeywell | 67-64-1 | −95 |
| Acetonitrile | 99.9% | Merck | 75-05-8 | −45.7 |
| DMSO | 99.9% | Merck | 67-68-5 | 18.5 |
| Formic acid | 100% | Merck | 64-18-6 | 4 |
| n-Butanol | 99.5% | Merck | 71-36-3 | −89 |
| n-Propanol | Alkaloid Skopje | 71-23-8 | −126.0 | |
| Ethanol (EtOH) | 99.9% | Carlo Erba | 64-17-5 | −114.1 |
| Methanol (MeOH) | 99.9% | Honeywell | 67-56-1 | −98 |
| H2O | Distilled semi-Q | 0.0 |
| Nonpolar | Polar | |
|---|---|---|
| Aprotic | Protic | |
| n-Hexane | Dichloromethane | Formic acid |
| Cyclohexane | THF | n-Butanol |
| Benzene | Ethyl acetate | n-Propanol |
| Toluene | Acetone | Ethanol |
| 1,4-Dioxane | Acetonitrile | Methanol |
| Diethyl ether | DMSO | H2O |
| Frequency (cm−1) | Band Assignment | Species |
|---|---|---|
| 929 | C−H out-of-plane deformation | Furfural |
| 1018 1081 | Ring deformation | |
| 1156 1279 | =C–O–C= ring vibration | |
| 1570 | C=C stretches | |
| 1696 1671 | C=O aldehyde stretch | |
| 1163 | C−O acid stretch vibration | Levulinic acid |
| 1369 1402 | CH3 symmetric and asymmetric bending vibration | |
| 1704 1742 | C=O aldehyde stretch | |
| 772−809 | C−H out of plane deformation | HMF |
| 1018 1070 | Ring deformation | |
| 1193 | =C–O–C= ring vibration | |
| 13,421−521 | C=C stretches | |
| 1663 | C=O aldehyde stretch |
| Solvent | Calculated Solubility of Furfural in 15 Different Solvents in g L−1 | Calculated Solubility of Levulinic Acid in 14 Different Solvents in g L−1 | Calculated Solubility of Hydroxymethylfurfural in 12 Different Solvents in g L−1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| −10 °C | 0 °C | 25 °C | −10 °C | 0 °C | 25 °C | −10 °C | 0 °C | 25 °C | |
| n-Hexane | IN | IN | IN | IN | IN | IN | IN | IN | IN |
| Cyclohexane | IN | IN | IN | IN | IN | IN | IN | IN | IN |
| Benzene | * | * | 570 ± 40 | * | * | 430 ± 30 | IN | IN | IN |
| Toluene | 430 ± 30 | 480 ± 30 | 610 ± 40 | 250 ± 20 | 450 ± 30 | 470 ± 30 | IN | IN | IN |
| 1,4-Dioxane | * | * | 1110 ± 80 | * | * | 1020 ± 70 | * | * | FM |
| Diethyl ether | 420 ± 30 | 1040 ± 70 | FM | 620 ± 40 | 620 ± 40 | 620 ± 40 | IN | IN | IN |
| Dichloromethane | 550 ± 40 | 670 ± 50 | 870 ± 60 | 500 ± 40 | 520 ± 40 | 570 ± 40 | 560 ± 40 | 590 ± 40 | 700 ± 50 |
| THF | 740 ± 50 | 760 ± 50 | FM | 940 ± 70 | FM | FM | 560 ± 40 | FM | FM |
| EtOAc | 850 ± 60 | 860 ± 60 | 1030 ± 70 | 400 ± 30 | 690 ± 50 | 850 ± 60 | 660 ± 50 | 660 ± 50 | 660 ± 50 |
| Acetone | 760 ± 50 | 810 ± 60 | 810 ± 60 | FM | FM | FM | FM | FM | FM |
| Acetonitrile | 910 ± 60 | 910 ± 60 | 910 ± 60 | FM | FM | FM | FM | FM | FM |
| DMSO | * | * | 820 ± 60 | * | * | 610 ± 40 | * | * | 660 ± 50 |
| Formic acid | DG | DG | DG | DG | DG | DG | DG | DG | DG |
| n-Butanol | 760 ± 50 | 810 ± 60 | 850 ± 60 | 840 ± 60 | 840 ± 60 | 840 ± 60 | 920 ± 60 | 920 ± 60 | 920 ± 60 |
| n-Propanol | 720 ± 50 | 810 ± 60 | 820 ± 60 | 610 ± 40 | 730 ± 50 | 800 ± 60 | 530 ± 40 | 530 ± 40 | 720 ± 50 |
| EtOH | 760 ± 50 | 840 ± 60 | 990 ± 70 | FM | 480 ± 30 | 930 ± 70 | 670 ± 50 | 800 ± 60 | 1030 ± 70 |
| MeOH | 690 ± 50 | 720 ± 50 | 960 ± 70 | 890 ± 60 | 890 ± 60 | 890 ± 60 | 580 ± 40 | 1020 ± 70 | FM |
| H2O | * | * | 90 ± 10 | * | * | NS | * | * | 180 ± 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakob, A.; Grilc, M.; Teržan, J.; Likozar, B. Solubility Temperature Dependence of Bio-Based Levulinic Acid, Furfural, and Hydroxymethylfurfural in Water, Nonpolar, Polar Aprotic and Protic Solvents. Processes 2021, 9, 924. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9060924
Jakob A, Grilc M, Teržan J, Likozar B. Solubility Temperature Dependence of Bio-Based Levulinic Acid, Furfural, and Hydroxymethylfurfural in Water, Nonpolar, Polar Aprotic and Protic Solvents. Processes. 2021; 9(6):924. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9060924
Chicago/Turabian StyleJakob, Ana, Miha Grilc, Janvit Teržan, and Blaž Likozar. 2021. "Solubility Temperature Dependence of Bio-Based Levulinic Acid, Furfural, and Hydroxymethylfurfural in Water, Nonpolar, Polar Aprotic and Protic Solvents" Processes 9, no. 6: 924. https://0-doi-org.brum.beds.ac.uk/10.3390/pr9060924