3.1. Response Surface Methodology
RSM is a statistical technique used for the estimation of relationships between input and response variables. It adopts linear, quadratic, or higher-order polynomial functions to investigate the statistical significance of the study factors and their interactions. Moreover, the regression concept is used for the prediction and optimization of responses. Over the years, the use of RSM in engineering fields has shown welcome results in the prediction of complex systems.
In the current study, the examined parameters were engine load and HHO concentration. Design-Expert version 11 was used for defining the multi-level historical design. The candidate set was created using user-defined discrete levels. Engine load and HHO concentration were assigned to four and six levels, respectively. The response variables measured were BTE, BSFC, CO, HC, noise, and smoke. The best fit model for each response was selected and analysis of variance (ANOVA) was applied for a better understanding of model attributes. In ANOVA, F is a probability distribution in different samplings, Df is degrees of freedom and the p-value is a statistical measure of variations in samples of a particular property. The decision rule for significance was benchmarked as a p-value less than 0.05. The percentage contribution (PC%) of each model term was calculated, which is a ratio of an aggregate of squared deviations to an individual sum of squares (SOS). PC% is a tool that provides a rough idea about the relative importance of study factors and the interactions.
3.2. ANOVA Results
The ANOVA results and fit statistics for BTE are presented in
Table 3. The F-value of 1980.51 and
p-value less than 0.0001 show that the model for BTE is significant. Moreover, the R
2 value of 0.9976 (refer to
Table 4) is close to positive unity and there is sufficient agreement between predicted and adjusted R
2. The
p values from the ANOVA table show that both load and concentration of HHO are significant. However, the load is significantly contributing to aggregated variations with a PC% of 84.4 compared to fuel concentration (3.5%). The best fitted quadratic model from the fit summary was selected owing to the poor fit and aliased nature of linear and cubic models, respectively. The actual regression equation for BTE is given by Equation (1).
The contour plot (see
Figure 2a) reveals the impact of load and fuel addition on BTE variation. The red color of the contour region advocates high engine BTE at high load and high HHO concentration. The color gradually shifted to red with an increase in HHO amount. The more explicit variation of response (BTE) is noticeable in
Figure 2b. The 3D surface plot shows the rising curve of BTE with positive moments along the load and fuel axes. The maximum thermal efficiency is observable at an engine load of 75% and a 10 L/min flow rate of HHO. The improvement in BTE with HHO enrichment is due to the complete combustion of diesel in the presence of hydroxy gas which resulted from higher mean effective pressure near TDC, owing to the faster flame travel in the case of hydrogen. The dark and light circles above and below the surface represent the experimental and predicted values, respectively. Furthermore, the accuracy of the given models could be assessed using certain diagnostics tests and graphs. In general, small deviations between experimental and predicted results are desirable for efficient models.
Figure 3 shows a comparison of actual and predicted BTE. The minimal deviations of predicted values from actual data sets are testimony to a good fit of the quadratic regression model.
ANOVA results for the second response variable, BSFC, are shown in
Table 5. The model is significant owing to an F value of 169.80, a
p-value less than the designated range, and R
2 (0.9725) close to 1, as indicated in
Table 6. The ANOVA findings show the significant effect of both load and HHO on fuel consumption. However, compared on a comparative scale, the load variations were found to have a greater impact on an engine than HHO concentration, as evidenced by PCs of 72.9% and 1.4%, respectively. The quadratic regression equation for BSFC on an actual scale is shown by Equation (2).
The effect of HHO addition and load on the fuel consumption trend of an engine is shown in
Figure 4a,b. The contour plot in
Figure 4a shows that fuel economy improved with successive addition of HHO to diesel at high loads. Moreover, it is also evident that, for the load range of 15–45%, there are more abrupt variations in BSFC compared to high loads, as indicated by a multi-color region. The response surface curve in
Figure 4b shows the decreasing increasing trend of BSFC with load and fuel concentration. The sudden lift in the curve at the culmination is due to increased fuel demand at a high load owing to ample friction resistance. The improved fuel economy with HHO enrichment is primarily because of the higher calorific value of HHO and efficient combustion due to the lean diesel–HHO–air mixture [
38,
39]. The comparison of predicted and actual BSFC, as given in
Figure 5, shows a bit of disorder data near the regression line. The disorderliness is due to the manual use of equipment in calculating BSFC. However, the deviations are not so large and therefore the model is acceptable.
Similar to performance, quadratic models for emissions were also analyzed using ANOVA. The defined model for CO emissions was significant as shown in
Table 7. The results revealed that both factors were significant; however, the percentage contribution of load to overall variations was greater compared to fuel concentration. Moreover, the R
2 value was 0.9819 (refer to
Table 8) and there was a reasonable agreement between adjusted and predicted R
2. In an attempt to see the accuracy of the selected model, the actual versus predicted description in
Figure 6 could be used as a model accuracy measuring tool. It is discernible from the figure that the data points are near to the linear regression line and deviations are negligible. The CO emission regression equation on a coded scale is given by Equation (3).
The variations in emissions of carbon monoxide with load and HHO concentrations are shown in
Figure 7a,b. The contour plot (
Figure 7a) provides a general illustration of the CO emission pattern of the engine subjected to various loads. The emissions are shown with the multi-color scheme, where blue stands for the minimum and red for the maximum. The response surface in
Figure 7b depicts the CO variations with load and HHO. The main root of carbon monoxide generation is the partial burning of fuel inside the engine. The addition of hydroxy gas not only reduces the carbon content but also facilitates complete combustion which consequently reduces the emissions [
40]. Therefore, a curve is seen to be following a decreasing trend in the presence of HHO.
Similarly,
Table 9 presents the ANOVA results of HC emission. The model selected and input variables are significant because of
p values less than 0.005. The coefficient of determination, the R
2 value, however, is shown in
Table 10. Engine load and HHO concentration had percentage contributions of 82.4% and 10.3% respectively. The comparison of actual and predicted HC emissions in
Figure 8 shows that the selected model is accurate. Equation (4) gives the predicting regression equation of HC emissions.
The detailed effect of varying factors on hydrocarbon emissions is shown in
Figure 9a,b. The addition of HHO reduced HC emissions for all concentrations and the minimum emissions were found to be for 10 L/min, as shown in
Figure 9a. Similarly, the response surface shows the emission variations of each fuel combination and is seen following a decreasing trend. The presence of hydroxy gas reduces HC, while carbon present in lubricating oil and primary diesel fuel is oxidized by excessive oxygen and high combustion temperatures inside the cylinder. Moreover, a relatively short quenching distance and a wider flammability range in the case of gaseous fuel have improved the engine performance in this regard [
41].
In addition to the performance and emission of an engine, factors of noise and smoke have also been considered. When the piston oscillates in the cylinder, it creates vibrations which consequently cause high noise levels. Moreover, when sudden ignition of fuel occurs inside the combustion chamber, it generates pressure waves that increase the intensity of the vibrations [
42]. The smoke is produced as the result of a rich air–fuel mixture and lubricant burning in the combustion chamber [
12].
Table 11 and
Table 12 present the ANOVA results for noise and smoke. The quadratic models and study factors for both responses were significant. Variations in noise would be more due to HHO concentration rather than load, as shown by percentage contributions of 13.19% and 74.30%. Similarly, the smoke model unveils that both load and fuel amount have a significant impact on smoke produced. Moreover, a model R
2 value close to one (based on
Table 13 and
Table 14) and actual versus predicted diagnostic descriptions (
Figure 10 and
Figure 11) evidenced the accuracy of the selected models. Equations (5) and (6) give the second-order regression equations of noise and smoke.
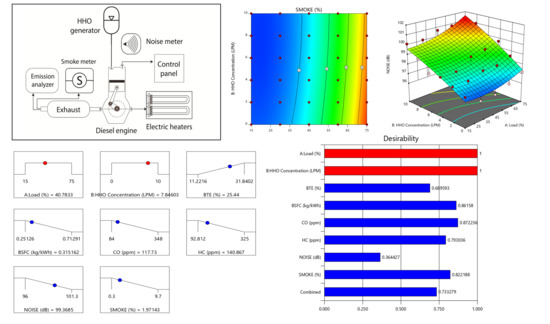
The effect of load and HHO on noise could be studied using the contour plots and response surface presented in
Figure 12a,b. The red-colored region at the right top corner of
Figure 12a indicates that, with the addition of HHO, the noise level increased and is at a maximum for 10 L/min HHO. The same trend could be seen more explicitly in
Figure 12b where the response surface shows the gradual increase in noise level. The increased noise level with the addition of hydroxy gas could be apprehended by improved thermal efficiency and excessive combustion at high pressures inside the chamber [
42,
43]. The opacity is seen following a decreasing trend with a rise in fuel enrichment and load, as shown by
Figure 13a,b. The contour plot and 3D response surface show that the least smoke is found for a blend of diesel with 10 L/min of HHO. The improved performance of an engine in terms of smoke emissions could be attributed to reduced HC emissions, high flame propagation, and high flame temperature of hydrogen [
44].