Anti-PD-1-Induced Hidradenitis Suppurativa
Abstract
:1. Introduction
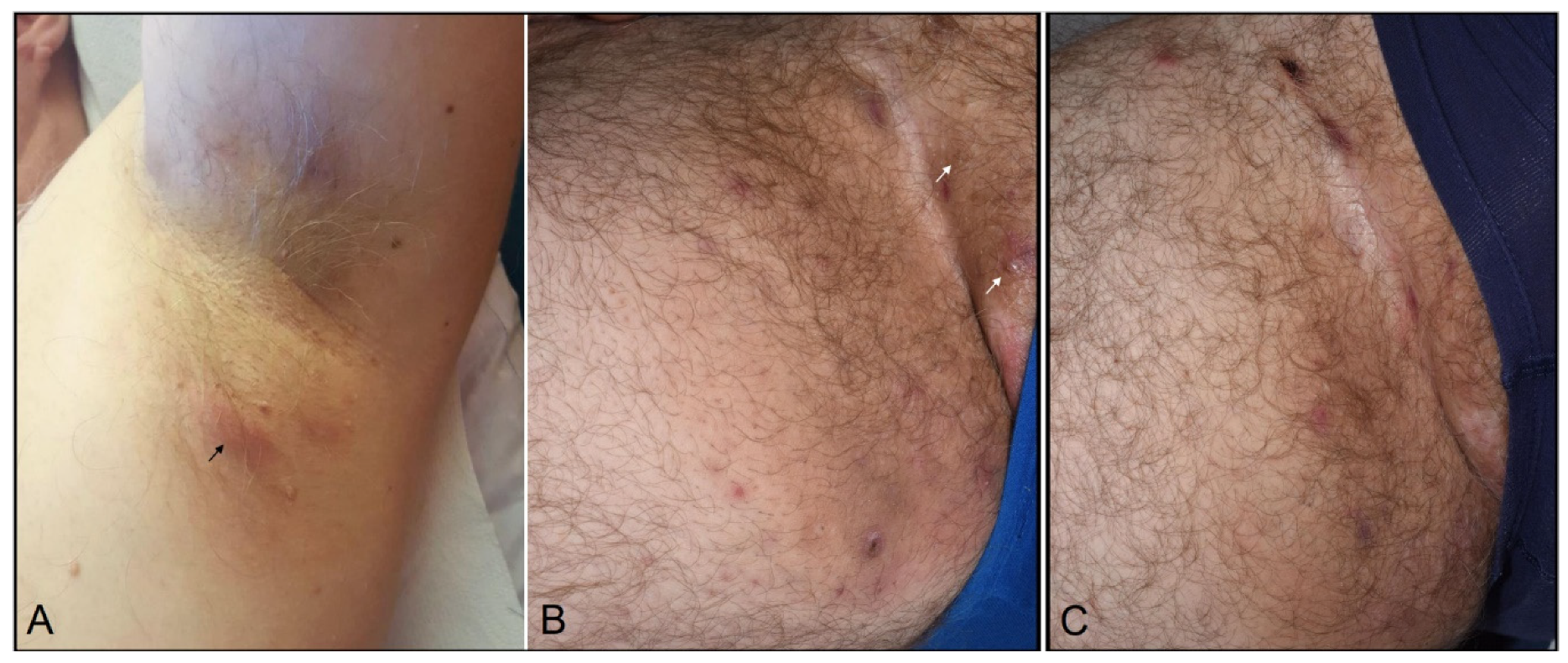
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Geisler, A.N.; Phillips, G.S.; Barrios, D.M.; Wu, J.; Leung, D.Y.M.; Moy, A.P.; Kern, J.A.; Lacouture, M.E. Immune checkpoint inhibitor-related dermatologic adverse events. J. Am. Acad. Dermatol. 2020, 83, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Xing, P.; Zhang, F.; Wang, G.; Xu, Y.; Li, C.; Wang, S.; Guo, Y.; Cai, S.; Wang, Y.; Li, J. Incidence rates of immune-related adverse events and their correlation with response in advanced solid tumours treated with NIVO or NIVO+IPI: A systematic review and meta-analysis. J. Immunother. Cancer 2019, 7, 341. [Google Scholar] [CrossRef] [PubMed]
- Bonigen, J.; Raynaud-Donzel, C.; Hureaux, J.; Kramkimel, N.; Blom, A.; Jeudy, G.; Breton, A.-L.; Hubiche, T.; Bedane, C.; Legoupil, D.; et al. Anti-PD1-induced psoriasis: A study of 21 patients. J. Eur. Acad. Dermatol. Venereol. 2017, 31, e254–e257. [Google Scholar] [CrossRef] [PubMed]
- Goldburg, S.R.; Strober, B.E.; Payette, M.J. Hidradenitis suppurativa: Epidemiology, clinical presentation, and pathogenesis. J. Am. Acad. Dermatol. 2020, 82, 1045–1058. [Google Scholar] [CrossRef]
- Seyed Jafari, S.M.; Hunger, R.E.; Schlapbach, C. Hidradenitis Suppurativa: Current understanding of pathogenic mechanisms and suggestion for treatment algorithm. Front Med. 2020, 7, 68. [Google Scholar] [CrossRef] [Green Version]
- Jemec, G.B.E.; Hansen, U. Histology of hidradenitis suppurativa. J. Am. Acad. Dermatol. 1995, 34, 994–999. [Google Scholar] [CrossRef]
- Ravi, V.; Maloney, N.J.; Worswick, S. Neutrophilic dermatoses as adverse effects of checkpoint inhibitors: A review. Dermatol. Ther. 2019, 32, e13074. [Google Scholar] [CrossRef] [PubMed]
- Ibraheim, H.; Perucha, E.; Powell, N. Pathology of immune-mediated tissue lesions following treatment with immune checkpoint inhibitors. Rheumatology 2019, 58 (Suppl. S7), vii17–vii28. [Google Scholar] [CrossRef] [Green Version]
- Moran, B.; Sweeney, C.M.; Hughes, R.; Malara, A.; Kirthi, S.; Tobin, A.-M.; Kirby, B.; Fletcher, J.M. Hidradenitis Suppurativa is characterized by dysregulation of the Th17: Treg cell axis, which is corrected by anti-TNF therapy. J. Investig. Dermatol. 2017, 137, 2389–2395. [Google Scholar] [CrossRef] [Green Version]
- Cătană, C.-S.; Berindan-Neagoe, I.; Cozma, V.; Magdaş, C.; Tăbăran, F.; Dumitraşcu, D.L. Contribution of the IL-17/IL-23 axis to the pathogenesis of inflammatory bowel disease. World J. Gastroenterol. 2015, 21, 5823–5830. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maillard, A.; Pastor, D.; Merat, R. Anti-PD-1-Induced Hidradenitis Suppurativa. Dermatopathology 2021, 8, 37-39. https://0-doi-org.brum.beds.ac.uk/10.3390/dermatopathology8010007
Maillard A, Pastor D, Merat R. Anti-PD-1-Induced Hidradenitis Suppurativa. Dermatopathology. 2021; 8(1):37-39. https://0-doi-org.brum.beds.ac.uk/10.3390/dermatopathology8010007
Chicago/Turabian StyleMaillard, Alexia, Damien Pastor, and Rastine Merat. 2021. "Anti-PD-1-Induced Hidradenitis Suppurativa" Dermatopathology 8, no. 1: 37-39. https://0-doi-org.brum.beds.ac.uk/10.3390/dermatopathology8010007





