5-Hydroxymethylcytosine Loss in Conjunctival Melanoma
Abstract
:1. Introduction
2. Material and Methods
2.1. Immunohistochemistry
2.2. In Situ Hybridization
2.3. Statistical Tests
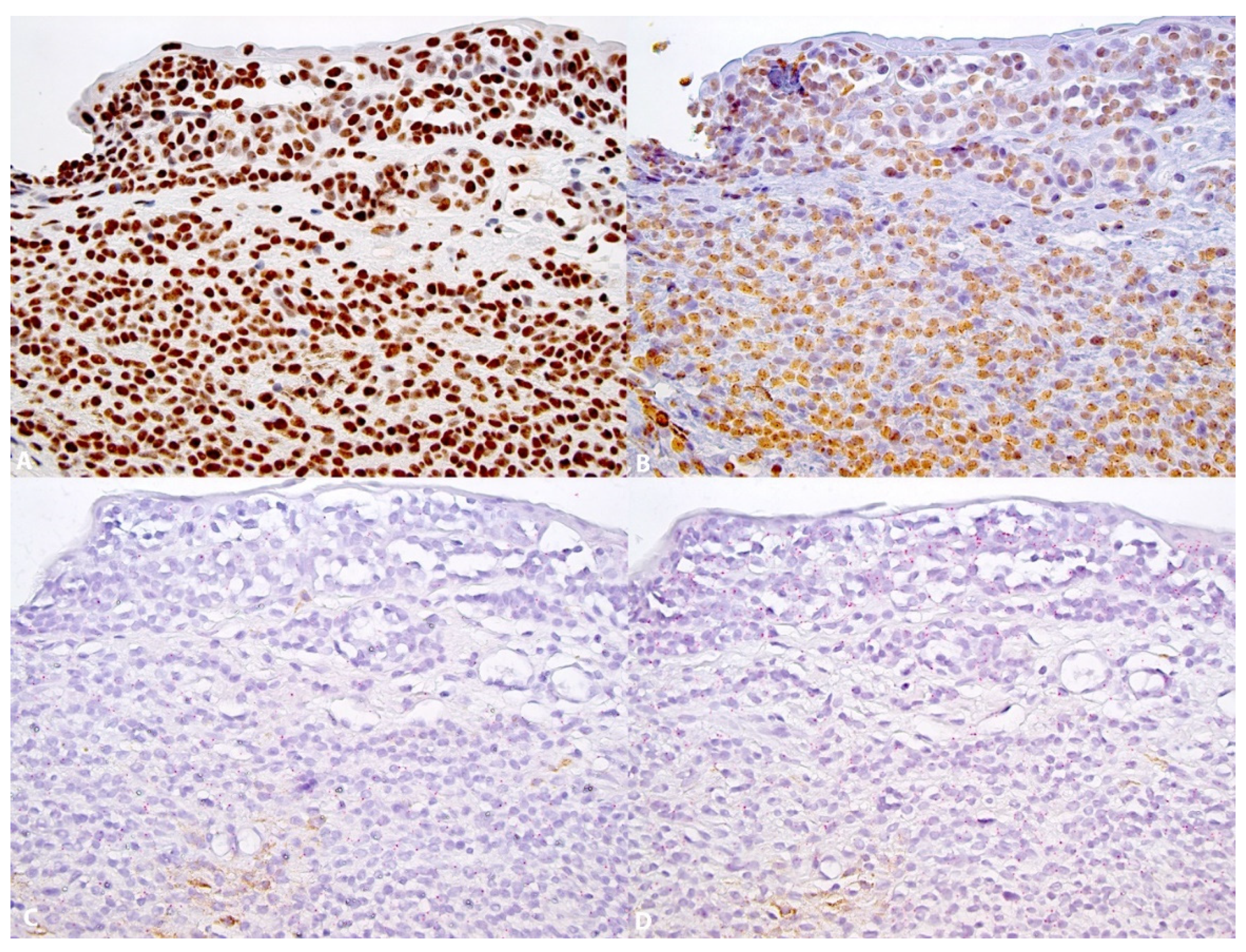
3. Results
3.1. Tumors and Patients
3.2. Expression of 5-hmC, 5-mC and TET2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, J.R.; Nanji, A.A.; Galor, A.; Karp, C.L. Management of conjunctival malignant melanoma: A review and update. Expert Rev. Ophthalmol. 2014, 9, 185–204. [Google Scholar] [CrossRef] [Green Version]
- Rossi, E.; Schinzari, G.; Maiorano, B.A.; Pagliara, M.M.; Di Stefani, A.; Bria, E.; Peris, K.; Blasi, M.A.; Tortora, G. Conjunctival Melanoma: Genetic and Epigenetic Insights of a Distinct Type of Melanoma. Int. J. Mol. Sci. 2019, 20, 5447. [Google Scholar] [CrossRef] [Green Version]
- Larsen, A.C. Conjunctival malignant melanoma in Denmark: Epidemiology, treatment and prognosis with special emphasis on tumorigenesis and genetic profile. Acta Ophthalmol. 2016, 94, 1–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastelan, S.; Gverovic Antunica, A.; Beketic Oreskovic, L.; Salopek Rabatic, J.; Kasun, B.; Bakija, I. Conjunctival Melanoma—Epidemiological Trends and Features. Pathol. Oncol. Res. 2018, 24, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.P.; Hu, D.N.; McCormick, S.; Finger, P.T. Conjunctival melanoma: Is it increasing in the United States? Am. J. Ophthalmol. 2003, 135, 800–806. [Google Scholar] [CrossRef]
- Hu, D.N.; Yu, G.; McCormick, S.A.; Finger, P.T. Population-based incidence of conjunctival melanoma in various races and ethnic groups and comparison with other melanomas. Am. J. Ophthalmol. 2008, 145, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.S.; Field, M.G.; Sant, D.; Wang, G.; Galor, A.; Dubovy, S.R.; Harbour, J.W.; Karp, C.L. Molecular Characteristics of Conjunctival Melanoma Using Whole-Exome Sequencing. JAMA Ophthalmol. 2017, 135, 1434–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larsen, A.C.; Dahmcke, C.M.; Dahl, C.; Siersma, V.D.; Toft, P.B.; Coupland, S.E.; Prause, J.U.; Guldberg, P.; Heegaard, S. A Retrospective Review of Conjunctival Melanoma Presentation, Treatment, and Outcome and an Investigation of Features Associated With BRAF Mutations. JAMA Ophthalmol. 2015, 133, 1295–1303. [Google Scholar] [CrossRef] [Green Version]
- Cisarova, K.; Folcher, M.; El Zaoui, I.; Pescini-Gobert, R.; Peter, V.G.; Royer-Bertrand, B.; Zografos, L.; Schalenbourg, A.; Nicolas, M.; Rimoldi, D.; et al. Genomic and transcriptomic landscape of conjunctival melanoma. PLoS Genet. 2020, 16, e1009201. [Google Scholar] [CrossRef]
- Scholz, S.L.; Cosgarea, I.; Susskind, D.; Murali, R.; Moller, I.; Reis, H.; Leonardelli, S.; Schilling, B.; Schimming, T.; Hadaschik, E.; et al. NF1 mutations in conjunctival melanoma. Br. J. Cancer 2018, 118, 1243–1247. [Google Scholar] [CrossRef]
- Rivolta, C.; Royer-Bertrand, B.; Rimoldi, D.; Schalenbourg, A.; Zografos, L.; Leyvraz, S.; Moulin, A. UV light signature in conjunctival melanoma; not only skin should be protected from solar radiation. J. Hum. Genet. 2016, 61, 361–362. [Google Scholar] [CrossRef] [Green Version]
- El Zaoui, I.; Bucher, M.; Rimoldi, D.; Nicolas, M.; Kaya, G.; Pescini Gobert, R.; Bedoni, N.; Schalenbourg, A.; Sakina, E.; Zografos, L.; et al. Conjunctival Melanoma Targeted Therapy: MAPK and PI3K/mTOR Pathways Inhibition. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2764–2772. [Google Scholar] [CrossRef] [Green Version]
- Lian, C.G.; Xu, Y.; Ceol, C.; Wu, F.; Larson, A.; Dresser, K.; Xu, W.; Tan, L.; Hu, Y.; Zhan, Q.; et al. Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell 2012, 150, 1135–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, S.; Wu, H.; Zhang, H.; Lian, C.G.; Lu, Q. DNA methylation/hydroxymethylation in melanoma. Oncotarget 2017, 8, 78163–78173. [Google Scholar] [CrossRef] [Green Version]
- Larson, A.R.; Dresser, K.A.; Zhan, Q.; Lezcano, C.; Woda, B.A.; Yosufi, B.; Thompson, J.F.; Scolyer, R.A.; Mihm, M.C., Jr.; Shi, Y.G.; et al. Loss of 5-hydroxymethylcytosine correlates with increasing morphologic dysplasia in melanocytic tumors. Mod. Pathol. 2014, 27, 936–944. [Google Scholar] [CrossRef] [Green Version]
- Uchiyama, R.; Uhara, H.; Uchiyama, A.; Ogawa, E.; Takazawa, Y.; Ashida, A.; Koga, H.; Hayashi, K.; Kiniwa, Y.; Okuyama, R. 5-Hydroxymethylcytosine as a useful marker to differentiate between malignant melanomas and benign melanocytic nevi. J. Dermatol. Sci. 2014, 73, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Rodic, N.; Zampella, J.; Sharma, R.; Burns, K.H.; Taube, J.M. Diagnostic utility of 5-hydroxymethylcytosine immunohistochemistry in melanocytic proliferations. J. Cutan. Pathol. 2015, 42, 807–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.J.; Li, L.M.; Zhang, R.H.; Xu, C.; Zhou, P.; Long, J.; Hu, G.; Jiang, M.J. The role of 5-hydroxymethylcytosine in melanoma. Melanoma Res. 2017, 27, 175–179. [Google Scholar] [CrossRef]
- Xu, W.; Yang, H.; Liu, Y.; Yang, Y.; Wang, P.; Kim, S.H.; Ito, S.; Yang, C.; Wang, P.; Xiao, M.T.; et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of alpha-ketoglutarate-dependent dioxygenases. Cancer Cell 2011, 19, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Gambichler, T.; Sand, M.; Skrygan, M. Loss of 5-hydroxymethylcytosine and ten-eleven translocation 2 protein expression in malignant melanoma. Melanoma Res. 2013, 23, 218–220. [Google Scholar] [CrossRef]
- Shibata, T.; Kokubu, A.; Miyamoto, M.; Sasajima, Y.; Yamazaki, N. Mutant IDH1 confers an in vivo growth in a melanoma cell line with BRAF mutation. Am. J. Pathol. 2011, 178, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Micevic, G.; Theodosakis, N.; Taube, J.M.; Bosenberg, M.W.; Rodic, N. Attenuation of genome-wide 5-methylcytosine level is an epigenetic feature of cutaneous malignant melanomas. Melanoma Res. 2017, 27, 85–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Diagnostic | Total n | M | F | Middle Age (SD) | Tumor Subtype | n |
|---|---|---|---|---|---|---|
| Nevi | 40 | 14 | 26 | 43.24 (23.54) | Compound nevus | 25 |
| Subepithelial nevus | 15 | |||||
| Melanoma | 37 | 17 | 20 | 67.89 (17.95) | De novo | 7 (18.9%) |
| Pre-existing nevus | 5 (13.5%) | |||||
| Pre-existing PAM | 25 (67.57%) |
| 5-mC | 5-hmC | TET2 | |
|---|---|---|---|
| Nevi n = 40 | |||
| Score 0 | 2 (5%) | ||
| Score 1 | 13 (32.5%) | 37 (92.5%) | |
| Score 2 | 22 (55%) | 1 (2.5%) | |
| Score 3 | 5 (12.5%) | 40 | |
| Melanomas n = 37 | |||
| Score 0 | 24 (64.9%) | ||
| Score 1 | 7 (18.9%) | 17 (46%) | 13 (35.1%) |
| Score 2 | 16 (43.2%) | 11 (29.7%) | |
| Score 3 | 14 (37.9%) | 9 (24.3%) |
| 5-hmC | 5-hmC | p | 5-mC | 5-mC | p | TET2 | TET2 | p | |
|---|---|---|---|---|---|---|---|---|---|
| P | A | P | A | P | A | ||||
| Age | |||||||||
| >65 | 10 | 10 | 16 | 4 | 8 | 12 | |||
| <65 | 10 | 7 | 0.743 1 | 14 | 3 | 1.0 1 | 5 | 12 | 0.730 1 |
| Sex | |||||||||
| Male | 11 | 6 | 15 | 2 | 5 | 12 | |||
| Female | 9 | 11 | 0.324 1 | 15 | 5 | 0.416 1 | 8 | 12 | 0.731 1 |
| Location | |||||||||
| Bulbar | 11 | 9 | 18 | 2 | 10 | 10 | |||
| Non-Bulb. | 9 | 8 | 1.0 1 | 12 | 5 | 0.212 1 | 3 | 14 | 0.0423 1 |
| Depth inv. | |||||||||
| >0.5 mm | 17 | 27 | 7 | 11 | 23 | ||||
| <0.5 mm | 3 | 17 | 0.234 1 | 3 | 0 | 1.0 1 | 2 | 1 | 0.277 1 |
| Ki67 | 23.7 | 35.9 | 0.0920 2 | 29,1 | 33 | 0.572 2 | 26.3 | 32.15 | 0.3668 2 |
| Ly. Inv. | |||||||||
| P | 17 | 8 | 7 | 4 | 3 | 8 | |||
| A | 3 | 9 | 0.0383 1 | 23 | 3 | 0.1630 1 | 10 | 16 | 0.7106 1 |
| TNM | |||||||||
| T1 | 9 | 7 | 14 | 2 | 9 | 7 | |||
| T2 | 7 | 3 | 9 | 1 | 3 | 7 | |||
| T3 | 4 | 7 | 0.2951 3 | 7 | 4 | 0.209 3 | 1 | 10 | 0.0384 3 |
| Recurrence | |||||||||
| P | 12 | 13 | 21 | 4 | 7 | 18 | |||
| A | 5 | 4 | 1.0 1 | 6 | 3 | 0.3482 1 | 5 | 4 | 0.224 1 |
| Origin | |||||||||
| Nevus | 3 | 2 | 4 | 1 | 2 | 3 | |||
| PAM | 14 | 11 | 21 | 4 | 6 | 19 | |||
| De novo | 3 | 4 | 5 | 2 | 5 | 2 | |||
| Death | |||||||||
| P | 3 | 3 | 4 | 2 | 6 | ||||
| A | 17 | 14 | 1.0 1 | 26 | 5 | 0.315 1 | 13 | 18 | 0.0719 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stahl, A.; Riggi, N.; Nardou, K.; Nicolas, M.; Kaya, G.; Moulin, A. 5-Hydroxymethylcytosine Loss in Conjunctival Melanoma. Dermatopathology 2021, 8, 176-184. https://0-doi-org.brum.beds.ac.uk/10.3390/dermatopathology8020023
Stahl A, Riggi N, Nardou K, Nicolas M, Kaya G, Moulin A. 5-Hydroxymethylcytosine Loss in Conjunctival Melanoma. Dermatopathology. 2021; 8(2):176-184. https://0-doi-org.brum.beds.ac.uk/10.3390/dermatopathology8020023
Chicago/Turabian StyleStahl, Alexandre, Nicolo Riggi, Katya Nardou, Michael Nicolas, Gurkan Kaya, and Alexandre Moulin. 2021. "5-Hydroxymethylcytosine Loss in Conjunctival Melanoma" Dermatopathology 8, no. 2: 176-184. https://0-doi-org.brum.beds.ac.uk/10.3390/dermatopathology8020023






