Concurrent Adjacent Merkel Cell Carcinoma and Chronic Lymphocytic Leukemia without Simultaneous Merkel Cell Polyomavirus Detection: A Case Series
Abstract
:1. Introduction
2. Materials and Methods
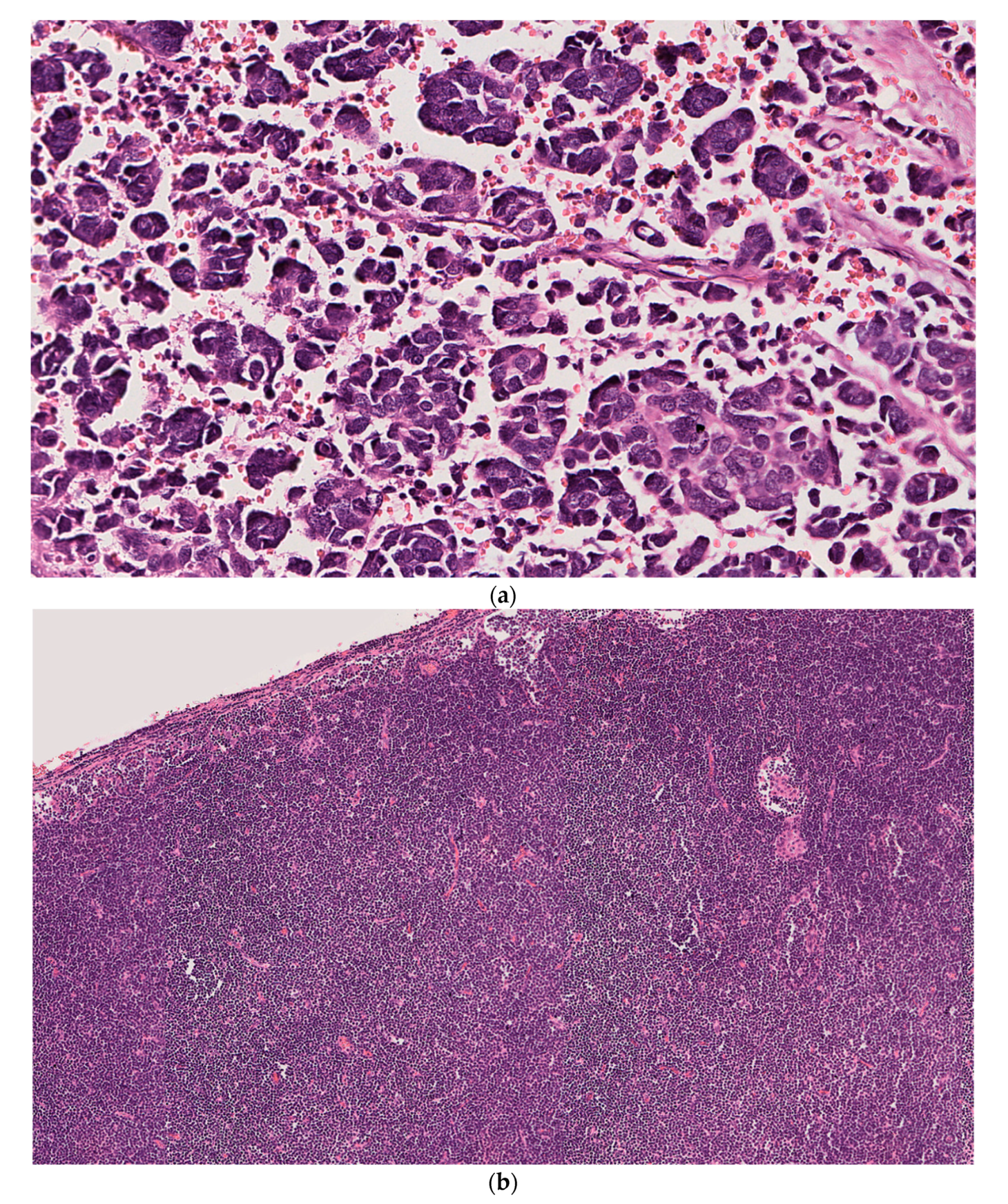
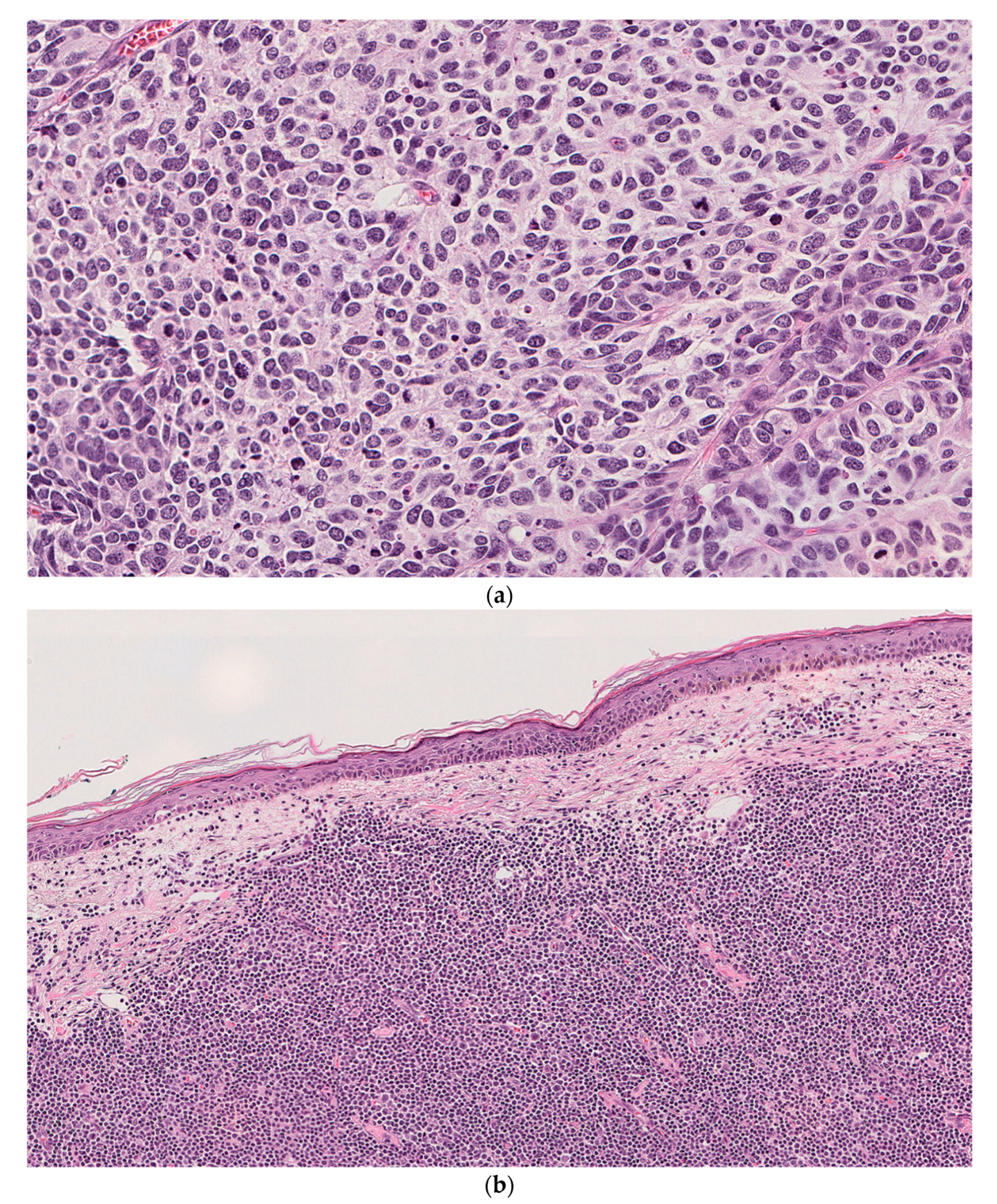
2.1. Case 1
2.2. Case 2
2.3. Case 3
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Agelli, M.; Clegg, L.X. Epidemiology of primary Merkel cell carcinoma in the United States. J. Am. Acad. Dermatol. 2003, 49, 832–841. [Google Scholar] [CrossRef]
- Albores-Saavedra, J.; Batich, K.; Chable-Montero, F.; Sagy, N.; Schwartz, A.M.; Henson, D.E. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: A population-based study. J. Cutan. Pathol. 2010, 37, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.; Moore, P.S. Merkel cell carcinoma: A virus-induced human cancer. Annu. Rev. Pathol. 2012, 7, 123–144. [Google Scholar] [CrossRef] [Green Version]
- Schrama, D.; Ugurel, S.; Becker, J.C. Merkel cell carcinoma: Recent insights and new treatment options. Curr. Opin. Oncol. 2012, 24, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Higaki-Mori, H.; Kuwamoto, S.; Iwasaki, T.; Kato, M.; Murakami, I.; Nagata, K.; Hayashi, K. Association of Merkel cell polyomavirus infection with clinicopathological differences in Merkel cell carcinoma. Hum. Pathol. 2012, 43, 2282–2291. [Google Scholar] [CrossRef] [PubMed]
- Sihto, H.; Kukko, H.; Koljonen, V.; Sankila, R.; Böhling, T.; Joensuu, H. Clinical factors associated with Merkel cell polyomavirus infection in Merkel cell carcinoma. J. Natl. Cancer Inst. 2009, 101, 938–945. [Google Scholar] [CrossRef]
- Schrama, D.; Peitsch, W.K.; Zapatka, M.; Kneitz, H.; Houben, R.; Eib, S.; Becker, J.C. Merkel cell polyomavirus status is not associated with clinical course of Merkel cell carcinoma. J. Investig. Dermatol. 2011, 131, 1631–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handschel, J.; Müller, D.; Depprich, R.A.; Ommerborn, M.A.; Kübler, N.R.; Naujoks, C.; Braunstein, S. The new polyomavirus (MCPyV) does not affect the clinical course in MCCs. Int. J. Oral. Maxillofac. Surg. 2010, 39, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Shuda, M.; Feng, H.; Kwun, H.J.; Rosen, S.T.; Gjoerup, O.; Moore, P.S.; Chang, Y. T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc. Natl. Acad. Sci. USA 2008, 105, 16272–16277. [Google Scholar] [CrossRef] [Green Version]
- Shuda, M.; Kwun, H.J.; Feng, H.; Chang, Y.; Moore, P.S. Human Merkel cell polyomavirus small T antigen is an oncoprotein targeting the 4E-BP1 translation regulator. J. Clin. Investig. 2011, 121, 3623–3634. [Google Scholar] [CrossRef]
- Kaae, J.; Hansen, A.V.; Biggar, R.J.; Boyd, H.A.; Moore, P.S.; Wohlfahrt, J.; Melbye, M. Merkel cell carcinoma: Incidence, mortality, and risk of other cancers. J. Natl. Cancer Inst. 2010, 102, 793–801. [Google Scholar] [CrossRef] [Green Version]
- Howard, R.A.; Dores, G.M.; Curtis, R.E.; Anderson, W.F.; Travis, L.B. Merkel cell carcinoma and multiple primary cancers. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1545–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teman, C.J.; Tripp, S.R.; Perkins, S.L.; Duncavage, E.J. Merkel cell polyomavirus (MCPyV) in chronic lymphocytic leukemia/small lymphocytic lymphoma. Leuk. Res. 2011, 35, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Pantulu, N.D.; Pallasch, C.P.; Kurz, A.K.; Kassem, A.; Frenzel, L.; Sodenkamp, S.; Zur Hausen, A. Detection of a novel truncating Merkel cell polyomavirus large T antigen deletion in chronic lymphocytic leukemia cells. Blood 2010, 116, 5280–5284. [Google Scholar] [CrossRef] [Green Version]
- Koljonen, V.; Kukko, H.; Pukkala, E.; Sankila, R.; Böhling, T.; Tukiainen, E.; Joensuu, H. Chronic lymphocytic leukaemia patients have a high risk of Merkel cell polyomavirus DNA-positive Merkel-cell carcinoma. Br. J. Cancer 2009, 101, 1444–1447. [Google Scholar] [CrossRef] [PubMed]
- Falchook, G.S.; Rady, P.; Hymes, S.; Nguyen, H.P.; Tyring, S.K.; Prieto, V.G.; Kurzrock, R. Merkel cell polyomavirus and HPV-17 associated with cutaneous squamous cell carcinoma arising in a patient with melanoma treated with the BRAF inhibitor dabrafenib. JAMA Dermatol. 2013, 149, 322–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paik, J.Y.; Hall, G.; Clarkson, A.; Lee, L.; Toon, C.; Colebatch, A.; Gill, A.J. Immunohistochemistry for Merkel cell polyomavirus is highly specific but not sensitive for the diagnosis of Merkel cell carcinoma in the Australian population. Hum. Pathol. 2011, 42, 1385–1390. [Google Scholar] [CrossRef]
- Sihto, H.; Kukko, H.; Koljonen, V.; Sankila, R.; Böhling, T.; Joensuu, H. Merkel cell polyomavirus infection, large T antigen, retinoblastoma protein and outcome in Merkel cell carcinoma. Clin. Cancer Res. 2011, 17, 4806–4813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waltari, M.; Sihto, H.; Kukko, H.; Koljonen, V.; Sankila, R.; Böhling, T.; Joensuu, H. Association of Merkel cell polyomavirus infection with tumor p53, KIT, stem cell factor, PDGFR-alpha and survival in Merkel cell carcinoma. Int. J. Cancer 2011, 129, 619–628. [Google Scholar] [CrossRef]
- Leonard, J.H.; Bell, J.R.; Kearsley, J.H. Characterization of cell lines established from Merkel-cell (‘‘small-cell’’) carcinoma of the skin. Int. J. Cancer 1993, 55, 803–810. [Google Scholar] [CrossRef]
- Pipas, J.M. Common and unique features of T antigens encoded by the polyomavirus group. J. Virol. 1992, 66, 3979–3985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houben, R.; Shuda, M.; Weinkam, R.; Schrama, D.; Feng, H.; Chang, Y.; Becker, J.C. Merkel cell polyomavirus-infected Merkel cell carcinoma cells require expression of viral T antigens. J. Virol. 2010, 84, 7064–7072. [Google Scholar] [CrossRef] [Green Version]
- Heath, M.; Jaimes, N.; Lemos, B.; Mostaghimi, A.; Wang, L.C.; Peñas, P.F.; Nghiem, P. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: The AEIOU features. J. Am. Acad. Dermatol. 2008, 58, 375–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulligan, S.P.; Tam, C.S. Chronic lymphocytic leukemia: Diagnosis and clinical staging. In Advances in the Treatment of B-Cell Chronic Lymphocytic Leukaemia; Keating, M.J., Tam, C.S., Eds.; Future Medicine: London, UK, 2012; pp. 6–15. [Google Scholar]
- Zur Hausen, H.; Gissmann, L. Lymphotropic papovaviruses isolated from African green monkey and human cells. Med. Microbiol. Immunol. 1979, 167, 137–153. [Google Scholar] [CrossRef] [PubMed]
- Ter Brugge, P.J.; Ta, V.B.; de Bruijn, M.J.; Keijzers, G.; Maas, A.; van Gent, D.C.; Hendriks, R.W. A mouse model for chronic lymphocytic leukemia based on expression of the SV40 large T antigen. Blood 2009, 114, 119–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cimino, P.J., Jr.; Bahler, D.W.; Duncavage, E.J. Detection of Merkel cell polyomavirus in chronic lymphocytic leukemia T-cells. Exp. Mol. Pathol. 2013, 94, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Paulson, K.G.; Iyer, J.G.; Blom, A.; Warton, E.M.; Sokil, M.; Yelistratova, L.; Nghiem, P. Systemic immune suppression predicts diminished Merkel cell carcinoma-specific survival independent of stage. J. Investig. Dermatol. 2013, 133, 642–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemminki, K.; Liu, X.; Ji, J.; Sundquist, J.; Sundquist, K. Kaposi sarcoma and Merkel cell carcinoma after autoimmune disease. Int. J. Cancer 2012, 131, E326–E328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanoy, E.; Engels, E.A. Skin cancers associated with autoimmune conditions among elderly adults. Br. J. Cancer 2010, 103, 112–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engels, E.A.; Frisch, M.; Goedert, J.J.; Biggar, R.J.; Miller, R.W. Merkel cell carcinoma and HIV infection. Lancet 2002, 359, 497–498. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.W.; Rabkin, C.S. Merkel cell carcinoma and melanoma: Etiological similarities and differences. Cancer Epidemiol. Biomark. Prev. 1999, 8, 153–158. [Google Scholar]
- Friedlaender, M.M.; Rubinger, D.; Rosenbaum, E.; Amir, G.; Siguencia, E. Temporary regression of Merkel cell carcinoma metastases after cessation of cyclosporine. Transplantation 2002, 73, 1849–1850. [Google Scholar] [CrossRef] [PubMed]




| MCPyV in MCC | MCPyV in CLL/SLL | |
|---|---|---|
| Patient 1 | Positive | Negative |
| Patient 2 | Negative | Negative |
| Patient 3 | Negative | Negative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saade, R.; Najjar, S.; Arslan, M.E.; Rady, P.; Tyring, S.K.; Nazeer, T. Concurrent Adjacent Merkel Cell Carcinoma and Chronic Lymphocytic Leukemia without Simultaneous Merkel Cell Polyomavirus Detection: A Case Series. Dermatopathology 2021, 8, 190-201. https://0-doi-org.brum.beds.ac.uk/10.3390/dermatopathology8020025
Saade R, Najjar S, Arslan ME, Rady P, Tyring SK, Nazeer T. Concurrent Adjacent Merkel Cell Carcinoma and Chronic Lymphocytic Leukemia without Simultaneous Merkel Cell Polyomavirus Detection: A Case Series. Dermatopathology. 2021; 8(2):190-201. https://0-doi-org.brum.beds.ac.uk/10.3390/dermatopathology8020025
Chicago/Turabian StyleSaade, Rayan, Saleh Najjar, Mustafa Erdem Arslan, Peter Rady, Stephen K. Tyring, and Tipu Nazeer. 2021. "Concurrent Adjacent Merkel Cell Carcinoma and Chronic Lymphocytic Leukemia without Simultaneous Merkel Cell Polyomavirus Detection: A Case Series" Dermatopathology 8, no. 2: 190-201. https://0-doi-org.brum.beds.ac.uk/10.3390/dermatopathology8020025






