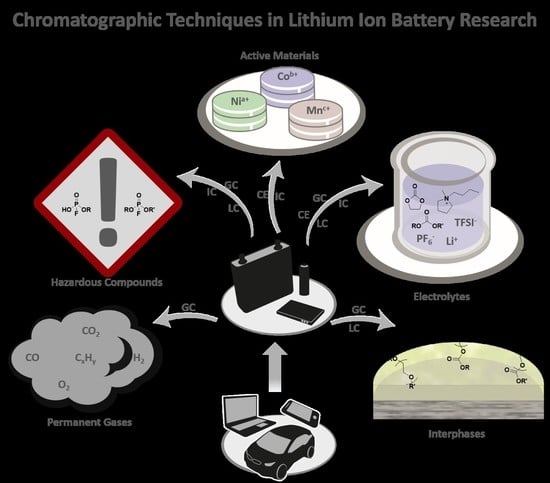
Chromatographic Techniques in the Research Area of Lithium Ion Batteries: Current State-of-the-Art
Abstract
:1. Introduction
2. Lithium Ion Batteries—Materials and Aging Effects
2.1. Lithium Ion Battery Materials and Composition
2.2. Lithium Ion Battery Aging and Degradation Mechanisms
3. Analysis of Lithium Ion Battery Materials
3.1. Electrode Analysis
3.2. Ionic Liquid Analysis
3.3. Non-Aqueous Electrolyte Analysis
3.3.1. Liquid Chromatography
3.3.2. Ion Chromatography
3.3.3. Pyrolysis-GC-MS
3.3.4. GC Investigations on Electrolyte Components
3.3.5. Permanent Gas Analysis
- a)
- Gases emerging during cell formation procedure
- b)
- Overcharge of the cells and related electrode/electrolyte reactions
- c)
- Elevated temperature conditions
- d)
- External abuse of the cells ultimately leading to thermal runaway
- a)
- Column parameters (stationary phase, length, inner diameter, coating thickness)
- b)
- Carrier gas and column flow
- c)
- Oven parameters (injector temperature, oven program, transfer line/interface temperature)
- d)
- Detector system and utilized parameters (e.g., temperature, gas flow, detector voltages, etc.)
- e)
- A chromatogram displaying the retention times of the investigated analytes
4. Further Literature
5. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AChE | acetylcholinesterase |
| AED | atomic emissions detector |
| AMIM+ | 1-amyl-3-methyl-imidazolium [cation] |
| ARC | accelerated rate calorimetry |
| BETI− | bis-perfluoroethylsulfonylimide [anion] |
| BMIM+ | 1-butyl-3-methyl-imidazolium [cation] |
| BOB− | bis-(oxalate)borate [anion] |
| CE | capillary electrophoresis |
| CEI | cathode electrolyte interphase |
| CZE | capillary zone electrophoresis |
| DCM | dichloromethane |
| DEC | diethyl carbonate |
| DEDOHC | diethyl-2,5-dioxahexane dicarboxylate |
| DFOB− | difluoro(oxalate)borate [anion] |
| DFP | difluoro phosphate |
| DMC | dimethyl carbonate |
| DMDOHC | dimethyl-2,5-dioxahexane dicarboxylate |
| DPC | diphenyl carbonate |
| EC | ethylene carbonate |
| EMC | ethyl methyl carbonate |
| EMDOHC | ethylmethyl-2,5-dioxahexane dicarboxylate |
| EMIM+ | 1-ethyl-3-methyl-imidazolium [cation] |
| EPC | ethyl phenyl carbonate |
| ES | ethylene sulfite |
| ESI | electrospray ionization |
| xEV | electric vehicle |
| FID | flame ionization detector |
| FSI− | bis(trifluoromethane)sulfonamide [anion] |
| FTIR | fourier transform infrared spectrometer |
| GC | gas chromatography |
| GPC | gel permeation chromatography |
| HILIC | hydrophilic interaction liquid chromatography |
| HS | headspace |
| IC | ion chromatography |
| ICP | inductively coupled plasma |
| IL | ionic liquid |
| ISE | ion selective electrode |
| IT | ion trap |
| LC | liquid chromatography |
| LCO | lithium cobalt oxide (LiCoO2) |
| LFP | lithium iron phosphate (LiFePO4) |
| LOD | limit of detection |
| LOQ | limit of quantification |
| LTO | lithium titanate (Li4Ti5O12) |
| MFP | monofluoro phosphate |
| MP | methyl propionate |
| MPC | methyl phenyl carbonate |
| MRM | multi reaction monitoring |
| MS | mass spectrometry |
| NCM | lithium nickel cobalt manganese oxide (LiNixCoyMnzO2; x + y + z = 1) |
| OEMS | online electrochemical mass spectrometry |
| OES | optical emissions spectroscopy |
| PC | propylene carbonate |
| PEG | polyethylene glycol |
| PMIM+ | 1-propyl-3-methyl-imidazolium [cation] |
| PNS | peripheral nervous system |
| PY | pyrolysis |
| PYR13+ | 1-methyl-1-propylpyrrolidinium [cation] |
| PYR14+ | 1-butyl-1-methylpyrrolidinium [cation] |
| QToF | quadrupole time-of-flight [mass spectrometer] |
| RP | reversed phase |
| RSD | relative standard deviation |
| SEI | solid electrolyte interphase |
| SFE | supercritical fluid extraction |
| SPME | solid phase microextraction |
| TCD | thermal conductivity detector |
| TFSI− | bis-(trifluoromethanesulfonyl)imide [anion] |
| TMD | transition metal dissolution |
| TPD | temperature-programmed decomposition |
| VC | vinylene carbonate |
| VUV | vacuum ultraviolet [detector] |
References
- Nishi, Y. Lithium ion secondary batteries; past 10 years and the future. J. Power Sources 2001, 100, 101–106. [Google Scholar] [CrossRef]
- Ozawa, K. Lithium-ion rechargeable batteries with LiCoO2 and carbon electrodes: The LiCoO2/C system. Solid State Ionics 1994, 69, 212–221. [Google Scholar] [CrossRef]
- Winter, M.; Barnett, B.; Xu, K. Before Li ion batteries. Chem. Rev. 2018, 118, 11433–11456. [Google Scholar] [CrossRef]
- Wagner, R.; Preschitschek, N.; Passerini, S.; Leker, J.; Winter, M. Current research trends and prospects among the various materials and designs used in lithium-based batteries. J. Appl. Electrochem. 2013, 43, 481–496. [Google Scholar] [CrossRef]
- Broussely, M.; Biensan, P.; Bonhomme, F.; Blanchard, P.; Herreyre, S.; Nechev, K.; Staniewicz, R. Main aging mechanisms in Li ion batteries. J. Power Sources 2005, 146, 90–96. [Google Scholar] [CrossRef]
- Schmuch, R.; Wagner, R.; Hörpel, G.; Placke, T.; Winter, M. Performance and cost of materials for lithium-based rechargeable automotive batteries. Nat. Energy 2018, 3, 267. [Google Scholar] [CrossRef]
- Placke, T.; Kloepsch, R.; Duehnen, S.; Winter, M. Lithium ion, lithium metal, and alternative rechargeable battery technologies: The odyssey for high energy density. J. Solid State Electrochem. 2017, 21, 1939–1964. [Google Scholar] [CrossRef]
- Bieker, P.; Winter, M. Lithium-Ionen-Technologie und was danach kommen könnte. Chem. Unserer Zeit 2016, 50, 172–186. [Google Scholar] [CrossRef]
- Andruleit, H.; Bahr, A.; Babies, H.; Franke, D.; Meßner, J.; Pierau, R.; Schauer, M.; Schmidt, S.; Weihmann, S. Energiestudie 2013. Reserven, Ressourcen und Verfügbarkeit von Energierohstoffen. Fachbereich B 2013, 1, 397–406. [Google Scholar]
- VDI Nachrichten, Technik & Wirtschaft; VDI Verlag GmbH: Düsseldorf, Germany, 2013; p. 34.
- Meister, P.; Jia, H.; Li, J.; Kloepsch, R.; Winter, M.; Placke, T. Best practice: Performance and cost evaluation of lithium ion battery active materials with special emphasis on energy efficiency. Chem. Mater. 2016, 28, 7203–7217. [Google Scholar] [CrossRef]
- Winter, M.; Moeller, K.-C.; Besenhard, J. Carbonaceous and graphitic anodes. In Lithium Batteries; Springer: Boston, MA, USA, 2009; pp. 145–194. [Google Scholar]
- Winter, M.; Besenhard, J.O. Lithiated carbons. In Handbook of Battery Materials; John Wiley & Sons: Hoboken, NJ, USA, 1999; pp. 383–418. [Google Scholar]
- Abraham, K.M. Directions in Secondary Lithium Battery Research-and-Development. Electrochim. Acta 1993, 38, 1233–1248. [Google Scholar] [CrossRef]
- Balbuena, P.B.; Wang, Y. Lithium-Ion Batteries: Solid-Electrolyte Interphase; World Scientific: Singapore, 2004; p. 424. [Google Scholar]
- Besenhard, J.O.; Winter, M. Insertion reactions in advanced electrochemical energy storage. Pure Appl. Chem. 1998, 70, 603–608. [Google Scholar] [CrossRef] [Green Version]
- Buqa, H.; Golob, P.; Winter, M.; Besenhard, J. Modified carbons for improved anodes in lithium ion cells. J. Power Sources 2001, 97, 122–125. [Google Scholar] [CrossRef]
- Buqa, H.; Blyth, R.; Golob, P.; Evers, B.; Schneider, I.; Alvarez, M.S.; Hofer, F.; Netzer, F.; Ramsey, M.; Winter, M. Negative electrodes in rechargeable lithium ion batteries—Influence of graphite surface modification on the formation of the solid electrolyte interphase. Ionics 2000, 6, 172–179. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohs, W.; Santner, H.; Hofer, F.; Schröttner, H.; Doninger, J.; Barsukov, I.; Buqa, H.; Albering, J.; Möller, K.-C.; Besenhard, J. A study on electrolyte interactions with graphite anodes exhibiting structures with various amounts of rhombohedral phase. J. Power Sources 2003, 119, 528–537. [Google Scholar] [CrossRef]
- Andre, D.; Kim, S.J.; Lamp, P.; Lux, S.F.; Maglia, F.; Paschos, O.; Stiaszny, B. Future generations of cathode materials: An automotive industry perspective. J. Mater. Chem. A 2015, 3, 6709–6732. [Google Scholar] [CrossRef]
- He, P.; Yu, H.; Zhou, H. Layered lithium transition metal oxide cathodes towards high energy lithium-ion batteries. J. Mater. Chem. 2012, 22, 3680–3695. [Google Scholar] [CrossRef]
- Börner, M.; Klamor, S.; Hoffmann, B.; Schroeder, M.; Nowak, S.; Würsig, A.; Winter, M.; Schappacher, F. Investigations on the C-Rate and Temperature Dependence of Manganese Dissolution/Deposition in LiMn2O4/Li4Ti5O12 Lithium Ion Batteries. J. Electrochem. Soc. 2016, 163, A831–A837. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Garche, J.; Dyer, C.K.; Moseley, P.T.; Ogumi, Z.; Rand, D.A.; Scrosati, B. Encyclopedia of Electrochemical Power Sources, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2013; p. 4538. [Google Scholar]
- Winter, M.; Besenhard, J.O.; Spahr, M.E.; Novák, P. Insertion electrode materials for rechargeable lithium batteries. Adv. Mater. 1998, 10, 725–763. [Google Scholar] [CrossRef]
- Winter, M.; Besenhard, J.O. Wiederaufladbare Batterien. Chem. Unserer Zeit 1999, 33, 252–266. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef]
- Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 2004, 104, 4303–4418. [Google Scholar] [CrossRef] [PubMed]
- Jow, T.R.; Xu, K.; Borodin, O.; Makoto, U. Electrolytes for Lithium and Lithium-Ion Batteries; Springer: Berlin, Germany, 2014; Volume 58. [Google Scholar]
- Tasaki, K.; Goldberg, A.; Winter, M. On the difference in cycling behaviors of lithium-ion battery cell between the ethylene carbonate-and propylene carbonate-based electrolytes. Electrochim. Acta 2011, 56, 10424–10435. [Google Scholar] [CrossRef]
- Ue, M. Mobility and Ionic Association of Lithium and Quaternary Ammonium Salts in Propylene Carbonate and γ-Butyrolactone. J. Electrochem. Soc. 1994, 141, 3336–3342. [Google Scholar] [CrossRef]
- Park, M.; Zhang, X.; Chung, M.; Less, G.B.; Sastry, A.M. A review of conduction phenomena in Li-ion batteries. J. Power Sources 2010, 195, 7904–7929. [Google Scholar] [CrossRef]
- Ahmadi, L.; Young, S.B.; Fowler, M.; Fraser, R.A.; Achachlouei, M.A. A cascaded life cycle: Reuse of electric vehicle lithium-ion battery packs in energy storage systems. Int. J. Life Cycle Assess. 2017, 22, 111–124. [Google Scholar] [CrossRef]
- Dewulf, J.; Van der Vorst, G.; Denturck, K.; Van Langenhove, H.; Ghyoot, W.; Tytgat, J.; Vandeputte, K. Recycling rechargeable lithium ion batteries: Critical analysis of natural resource savings. Resour. Conserv. Recycle 2010, 54, 229–234. [Google Scholar] [CrossRef]
- Xu, J.; Thomas, H.; Francis, R.W.; Lum, K.R.; Wang, J.; Liang, B. A review of processes and technologies for the recycling of lithium-ion secondary batteries. J. Power Sources 2008, 177, 512–527. [Google Scholar] [CrossRef]
- Vetter, J.; Novák, P.; Wagner, M.; Veit, C.; Möller, K.-C.; Besenhard, J.; Winter, M.; Wohlfahrt-Mehrens, M.; Vogler, C.; Hammouche, A. Ageing mechanisms in lithium-ion batteries. J. Power Sources 2005, 147, 269–281. [Google Scholar] [CrossRef]
- Lux, S.; Lucas, I.; Pollak, E.; Passerini, S.; Winter, M.; Kostecki, R. The mechanism of HF formation in LiPF6 based organic carbonate electrolytes. Electrochem. Commun. 2012, 14, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Ravdel, B.; Abraham, K.; Gitzendanner, R.; DiCarlo, J.; Lucht, B.; Campion, C. Thermal stability of lithium-ion battery electrolytes. J. Power Sources 2003, 119, 805–810. [Google Scholar] [CrossRef]
- Campion, C.L.; Li, W.; Lucht, B.L. Thermal decomposition of LiPF6-based electrolytes for lithium-ion batteries. J. Electrochem. Soc. 2005, 152, A2327–A2334. [Google Scholar] [CrossRef]
- Campion, C.L.; Li, W.; Euler, W.B.; Lucht, B.L.; Ravdel, B.; DiCarlo, J.F.; Gitzendanner, R.; Abraham, K. Suppression of toxic compounds produced in the decomposition of lithium-ion battery electrolytes. Electrochem. Solid-State Lett. 2004, 7, A194–A197. [Google Scholar] [CrossRef]
- Wilken, S.; Treskow, M.; Scheers, J.; Johansson, P.; Jacobsson, P. Initial stages of thermal decomposition of LiPF 6-based lithium ion battery electrolytes by detailed Raman and NMR spectroscopy. RSC Adv. 2013, 3, 16359–16364. [Google Scholar] [CrossRef]
- Tripathi, H.L.; Dewey, W.L. Comparison of the effects of diisopropylfluorophosphate, sarin, soman, and tabun on toxicity and brain acetylcholinesterase activity in mice. J. Toxicol. Environ. Health 1989, 26, 437–446. [Google Scholar] [CrossRef]
- Silver, S. The Toxieity of Dimethyl-, Diethyl-, and Diisopropyl Fluorophosphate Vapors. J. Ind. Hygiene Toxicol. 1948, 30, 307–311. [Google Scholar]
- Costa, L.G. Current issues in organophosphate toxicology. Clin. Chim. Acta 2006, 366, 1–13. [Google Scholar] [CrossRef]
- Raushel, F.M. Chemical biology: Catalytic detoxification. Nature 2011, 469, 310–311. [Google Scholar] [CrossRef]
- Nowak, S.; Winter, M. Review—Chemical Analysis for a Better Understanding of Aging and Degradation Mechanisms of Non-Aqueous Electrolytes for Lithium Ion Batteries: Method Development, Application and Lessons Learned. J. Electrochem. Soc. 2015, 162, A2500–A2508. [Google Scholar] [CrossRef]
- Weber, W.; Wagner, R.; Streipert, B.; Kraft, V.; Winter, M.; Nowak, S. Ion and gas chromatography mass spectrometry investigations of organophosphates in lithium ion battery electrolytes by electrochemical aging at elevated cathode potentials. J. Power Sources 2016, 306, 193–199. [Google Scholar] [CrossRef]
- Kraft, V.; Grützke, M.; Weber, W.; Winter, M.; Nowak, S. Ion chromatography electrospray ionization mass spectrometry method development and investigation of lithium hexafluorophosphate-based organic electrolytes and their thermal decomposition products. J. Chromatogr. A 2014, 1354, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Kraft, V.; Grützke, M.; Weber, W.; Menzel, J.; Wiemers-Meyer, S.; Winter, M.; Nowak, S. Two-dimensional ion chromatography for the separation of ionic organophosphates generated in thermally decomposed lithium hexafluorophosphate-based lithium ion battery electrolytes. J. Chromatogr. A 2015, 1409, 201–209. [Google Scholar] [CrossRef]
- Grützke, M.; Kraft, V.; Hoffmann, B.; Klamor, S.; Diekmann, J.; Kwade, A.; Winter, M.; Nowak, S. Aging investigations of a lithium-ion battery electrolyte from a field-tested hybrid electric vehicle. J. Power Sources 2015, 273, 83–88. [Google Scholar] [CrossRef]
- Sasaki, T.; Abe, T.; Iriyama, Y.; Inaba, M.; Ogumi, Z. Suppression of an alkyl dicarbonate formation in li-ion cells. J. Electrochem. Soc. 2005, 152, A2046–A2050. [Google Scholar] [CrossRef]
- Sasaki, T.; Abe, T.; Iriyama, Y.; Inaba, M.; Ogumi, Z. Formation mechanism of alkyl dicarbonates in Li-ion cells. J. Power Sources 2005, 150, 208–215. [Google Scholar] [CrossRef]
- Gachot, G.; Grugeon, S.; Eshetu, G.G.; Mathiron, D.; Ribière, P.; Armand, M.; Laruelle, S. Thermal behaviour of the lithiated-graphite/electrolyte interface through GC/MS analysis. Electrochim. Acta 2012, 83, 402–409. [Google Scholar] [CrossRef]
- Schultz, C.; Vedder, S.; Winter, M.; Nowak, S. Qualitative Investigation of the Decomposition of Organic Solvent Based Lithium Ion Battery Electrolytes with LC-IT-TOF-MS. Anal. Chem. 2016, 88, 11160–11168. [Google Scholar] [CrossRef]
- Schultz, C.; Kraft, V.; Pyschik, M.; Weber, S.; Schappacher, F.; Winter, M.; Nowak, S. Separation and Quantification of Organic Electrolyte Components in Lithium-Ion Batteries via a Developed HPLC Method. J. Electrochem. Soc. 2015, 162, A629–A634. [Google Scholar] [CrossRef]
- Yoshida, H.; Fukunaga, T.; Hazama, T.; Terasaki, M.; Mizutani, M.; Yamachi, M. Degradation mechanism of alkyl carbonate solvents used in lithium-ion cells during initial charging. J. Power Sources 1997, 68, 311–315. [Google Scholar] [CrossRef]
- Winter, M. The Solid Electrolyte Interphase - The Most Important and the Least Understood Solid Electrolyte in Rechargeable Li Batteries. Zeitschrift Fur Physikalische Chemie 2009, 223, 1395–1406. [Google Scholar] [CrossRef]
- Schwieters, T.; Evertz, M.; Mense, M.; Winter, M.; Nowak, S. Lithium loss in the solid electrolyte interphase: Lithium quantification of aged lithium ion battery graphite electrodes by means of laser ablation inductively coupled plasma mass spectrometry and inductively coupled plasma optical emission spectroscopy. J. Power Sources 2017, 356, 47–55. [Google Scholar] [CrossRef]
- Peled, E. The electrochemical behavior of alkali and alkaline earth metals in nonaqueous battery systems—the solid electrolyte interphase model. J. Electrochem. Soc. 1979, 126, 2047–2051. [Google Scholar] [CrossRef]
- Dey, A. Lithium anode film and organic and inorganic electrolyte batteries. Thin Solid Films 1977, 43, 131–171. [Google Scholar] [CrossRef]
- Nordh, T.; Younesi, R.; Hahlin, M.; Duarte, R.F.; Tengstedt, C.; Brandell, D.; Edstrom, K. Manganese in the SEI Layer of Li4Ti5O12 Studied by Combined NEXAFS and HAXPES Techniques. J. Phys. Chem. C 2016, 120, 3206–3213. [Google Scholar] [CrossRef]
- Nitta, N.; Benson, J.; Lee, J.T.; Kovalenko, I.; Tighe, S.; Fuller, T.F.; Yushin, G. A Combined Investigation into the Effect of Ni/Mn/Co Ions on Lithium-Ion Battery Anodes Using X-Ray Photoelectron Spectroscopy and Secondary Ion Mass Spectrometry. ECS Meeting Abstracts 2014, 245. [Google Scholar]
- Shin, H.; Park, J.; Sastry, A.M.; Lu, W. Degradation of the solid electrolyte interphase induced by the deposition of manganese ions. J. Power Sources 2015, 284, 416–427. [Google Scholar] [CrossRef]
- Joshi, T.; Eom, K.; Yushin, G.; Fuller, T.F. Effects of dissolved transition metals on the electrochemical performance and SEI growth in lithium-ion batteries. J. Electrochem. Soc. 2014, 161, A1915–A1921. [Google Scholar] [CrossRef]
- Delacourt, C.; Kwong, A.; Liu, X.; Qiao, R.; Yang, W.; Lu, P.; Harris, S.; Srinivasan, V. Effect of manganese contamination on the solid-electrolyte-interphase properties in Li-ion batteries. J. Electrochem. Soc. 2013, 160, A1099–A1107. [Google Scholar] [CrossRef]
- Würsig, A.; Buqa, H.; Holzapfel, M.; Krumeich, F.; Novák, P. Film formation at positive electrodes in lithium-Ion batteries. Electrochem. Solid-State Lett. 2005, 8, A34–A37. [Google Scholar] [CrossRef]
- Kim, J.-M.; Chung, H.-T. The first cycle characteristics of Li [Ni 1/3 Co 1/3 Mn 1/3] O 2 charged up to 4.7 V. Electrochim. Acta 2004, 49, 937–944. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Lee, K.-S.; Sun, Y.-K.; Lee, Y.M.; Kim, D.-W. Effect of an organic additive on the cycling performance and thermal stability of lithium-ion cells assembled with carbon anode and LiNi 1/3 Co 1/3 Mn 1/3 O 2 cathode. J. Power Sources 2011, 196, 6997–7001. [Google Scholar] [CrossRef]
- Qian, Y.; Schultz, C.; Niehoff, P.; Schwieters, T.; Nowak, S.; Schappacher, F.M.; Winter, M. Investigations on the electrochemical decomposition of the electrolyte additive vinylene carbonate in Li metal half cells and lithium ion full cells. J. Power Sources 2016, 332, 60–71. [Google Scholar] [CrossRef]
- Qian, Y.; Niehoff, P.; Börner, M.; Grützke, M.; Mönnighoff, X.; Behrends, P.; Nowak, S.; Winter, M.; Schappacher, F.M. Influence of electrolyte additives on the cathode electrolyte interphase (CEI) formation on LiNi1/3Mn1/3Co1/3O2 in half cells with Li metal counter electrode. J. Power Sources 2016, 329, 31–40. [Google Scholar] [CrossRef]
- Antolini, E. LiCoO 2: Formation, structure, lithium and oxygen nonstoichiometry, electrochemical behaviour and transport properties. Solid State Ionics 2004, 170, 159–171. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Kang, S.H.; Johnson, C.S.; Vaughey, J.T.; Benedek, R.; Hackney, S.A. Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J. Mater. Chem. 2007, 17, 3112. [Google Scholar] [CrossRef]
- Liu, X.; Liu, J.; Qiao, R.; Yu, Y.; Li, H.; Suo, L.; Hu, Y.-s.; Chuang, Y.-D.; Shu, G.; Chou, F.; et al. Phase Transformation and Lithiation Effect on Electronic Structure of LixFePO4: An In-Depth Study by Soft X-ray and Simulations. J. Am. Chem. Soc. 2012, 134, 13708–13715. [Google Scholar] [CrossRef] [Green Version]
- Goodenough, J.; Mizushima, K.; Takeda, T. Solid-solution oxides for storage-battery electrodes. Jpn. J. Appl. Phys. 1980, 19, 305. [Google Scholar] [CrossRef]
- Krueger, S.; Kloepsch, R.; Li, J.; Nowak, S.; Passerini, S.; Winter, M. How do reactions at the anode/electrolyte interface determine the cathode performance in lithium-ion batteries? J. Electrochem. Soc. 2013, 160, A542–A548. [Google Scholar] [CrossRef]
- Ohzuku, T.; Makimura, Y. Layered lithium insertion material of LiCo1/3Ni1/3Mn1/3O2 for lithium-ion batteries. Chem. Lett. 2001, 30, 642–643. [Google Scholar] [CrossRef]
- Hu, M.; Pang, X.; Zhou, Z. Recent progress in high-voltage lithium ion batteries. J. Power Sources 2013, 237, 229–242. [Google Scholar] [CrossRef]
- Zheng, H.; Sun, Q.; Liu, G.; Song, X.; Battaglia, V.S. Correlation between dissolution behavior and electrochemical cycling performance for LiNi 1/3 Co 1/3 Mn 1/3 O 2-based cells. J. Power Sources 2012, 207, 134–140. [Google Scholar] [CrossRef]
- Jia, H.P.; Kloepsch, R.; He, X.; Evertz, M.; Nowak, S.; Li, J.; Winter, M.; Placke, T. Nanostructured ZnFe2O4 as Anode Material for Lithium-Ion Batteries: Ionic Liquid-Assisted Synthesis and Performance Evaluation with Special Emphasis on Comparative Metal Dissolution. Acta Chim. Slov. 2016, 63, 470–483. [Google Scholar] [CrossRef] [Green Version]
- Goriparti, S.; Miele, E.; De Angelis, F.; Di Fabrizio, E.; Proietti Zaccaria, R.; Capiglia, C. Review on recent progress of nanostructured anode materials for Li-ion batteries. J. Power Sources 2014, 257, 421–443. [Google Scholar] [CrossRef] [Green Version]
- Qian, Y.; Niehoff, P.; Zhou, D.; Adam, R.; Mikhailova, D.; Pyschik, M.; Börner, M.; Klöpsch, R.; Rafaja, D.; Schumacher, G. Investigation of nano-sized Cu (II) O as a high capacity conversion material for Li-metal cells and lithium-ion full cells. J. Mater. Chem. A 2017, 5, 6556–6568. [Google Scholar] [CrossRef]
- Novák, P.; Goers, D.; Spahr, M. Carbon Materials in Lithium-Ion Batteries. In Carbons for Electrochemical Energy Storage and Conversion Systems; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Endo, M.; Kim, Y.; Park, K. Advanced Battery Applications of Carbons. In Carbons for Electrochemical Energy Storage and Conversion Systems; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Brodd, R. Carbon in Batteries and Energy Conversion Devices. In Carbons for Electrochemical Energy Storage and Conversion Systems; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Flandrois, S.; Simon, B. Carbon materials for lithium-ion rechargeable batteries. Carbon 1999, 37, 165–180. [Google Scholar] [CrossRef]
- Schmitz, R.W.; Murmann, P.; Schmitz, R.; Müller, R.; Krämer, L.; Kasnatscheew, J.; Isken, P.; Niehoff, P.; Nowak, S.; Röschenthaler, G.-V. Investigations on novel electrolytes, solvents and SEI additives for use in lithium-ion batteries: Systematic electrochemical characterization and detailed analysis by spectroscopic methods. Prog. Solid State Chem. 2014, 42, 65–84. [Google Scholar] [CrossRef]
- Newman, G.; Francis, R.; Gaines, L.; Rao, B. Hazard investigations of LiClO4/dioxolane electrolyte. J. Electrochem. Soc. 1980, 127, 2025–2027. [Google Scholar] [CrossRef]
- Aurbach, D.; Talyosef, Y.; Markovsky, B.; Markevich, E.; Zinigrad, E.; Asraf, L.; Gnanaraj, J.S.; Kim, H.-J. Design of electrolyte solutions for Li and Li-ion batteries: A review. Electrochim. Acta 2004, 50, 247–254. [Google Scholar] [CrossRef]
- Ding, M.S.; Xu, K.; Jow, T.R. Conductivity and Viscosity of PC-DEC and PC-EC Solutions of LiBOB. J. Electrochem. Soc. 2005, 152, A132–A140. [Google Scholar] [CrossRef]
- Schedlbauer, T.; Krüger, S.; Schmitz, R.; Schmitz, R.W.; Schreiner, C.; Gores, H.J.; Passerini, S.; Winter, M. Lithium difluoro(oxalato)borate: A promising salt for lithium metal based secondary batteries? Electrochim. Acta 2013, 92, 102–107. [Google Scholar] [CrossRef]
- Böttcher, T.; Duda, B.; Kalinovich, N.; Kazakova, O.; Ponomarenko, M.; Vlasov, K.; Winter, M.; Röschenthaler, G.V. Syntheses of novel delocalized cations and fluorinated anions, new fluorinated solvents and additives for lithium ion batteries. Prog. Solid State Chem. 2014, 42, 202–217. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on electrolyte additives for lithium-ion batteries. J. Power Sources 2006, 162, 1379–1394. [Google Scholar] [CrossRef]
- Murmann, P.; Mönnighoff, X.; von Aspern, N.; Janssen, P.; Kalinovich, N.; Shevchuk, M.; Kazakova, O.; Röschenthaler, G.-V.; Cekic-Laskovic, I.; Winter, M. Influence of the fluorination degree of organophosphates on flammability and electrochemical performance in lithium ion batteries: Studies on fluorinated compounds deriving from triethyl phosphate. J. Electrochem. Soc. 2016, 163, A751–A757. [Google Scholar] [CrossRef]
- Dagger, T.; Rad, B.R.; Schappacher, F.M.; Winter, M. Comparative Performance Evaluation of Flame Retardant Additives for Lithium Ion Batteries–I. Safety, Chemical and Electrochemical Stabilities. Energy Technol. 2018, 6, 2011–2022. [Google Scholar] [CrossRef]
- Dagger, T.; Niehoff, P.; Lürenbaum, C.; Schappacher, F.M.; Winter, M. Comparative Performance Evaluation of Flame Retardant Additives for Lithium Ion Batteries–II. Full Cell Cycling and Postmortem Analyses. Energy Technol. 2018, 6, 2023–2035. [Google Scholar] [CrossRef]
- Winter, M.; Imhof, R.; Joho, F.; Novak, P. FTIR and DEMS investigations on the electroreduction of chloroethylene carbonate-based electrolyte solutions for lithium-ion cells. J. Power Sources 1999, 81, 818–823. [Google Scholar] [CrossRef]
- Tobishima, S.; Ogino, Y.; Watanabe, Y. Influence of electrolyte additives on safety and cycle life of rechargeable lithium cells. J. Appl. Electrochem. 2003, 33, 143–150. [Google Scholar] [CrossRef]
- Shigematsu, Y.; Kinoshita, S.-i.; Ue, M. Thermal behavior of a C/ LiCoO2 cell, its components, and their combinations and the effects of electrolyte additives. J. Electrochem. Soc. 2006, 153, A2166–A2170. [Google Scholar] [CrossRef]
- Möller, K.-C.; Santner, H.; Kern, W.; Yamaguchi, S.; Besenhard, J.; Winter, M. In situ characterization of the SEI formation on graphite in the presence of a vinylene group containing film-forming electrolyte additives. J. Power Sources 2003, 119, 561–566. [Google Scholar] [CrossRef]
- Korepp, C.; Santner, H.; Fujii, T.; Ue, M.; Besenhard, J.; Möller, K.-C.; Winter, M. 2-Cyanofuran—A novel vinylene electrolyte additive for PC-based electrolytes in lithium-ion batteries. J. Power Sources 2006, 158, 578–582. [Google Scholar] [CrossRef]
- Santner, H.; Möller, K.-C.; Ivančo, J.; Ramsey, M.; Netzer, F.P.; Yamaguchi, S.; Besenhard, J.; Winter, M. Acrylic acid nitrile, a film-forming electrolyte component for lithium-ion batteries, which belongs to the family of additives containing vinyl groups. J. Power Sources 2003, 119, 368–372. [Google Scholar] [CrossRef]
- Schranzhofer, H.; Bugajski, J.; Santner, H.J.; Korepp, C.; Möller, K.C.; Besenhard, J.O.; Winter, M.; Sitte, W. Electrochemical impedance spectroscopy study of the SEI formation on graphite and metal electrodes. J. Power Sources 2006, 153, 391. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Nicolau, B.G.; Esbenshade, J.L.; Gewirth, A.A. Characterization of the Cathode Electrolyte Interface in Lithium Ion Batteries by Desorption Electrospray Ionization Mass Spectrometry. Anal. Chem. 2016, 88, 7171–7177. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.G.S.R.; Bruce, P.G.; Goodenough, J.B. AC Impedance Analysis of Polycrystalline Insertion Electrodes: Application to Li1 − x CoO2. J. Electrochem. Soc. 1985, 132, 1521–1528. [Google Scholar] [CrossRef]
- Fung, Y.S.; Zhou, R.Q. Room temperature molten salt as medium for lithium battery. J. Power Sources 1999, 81–82, 891–895. [Google Scholar] [CrossRef]
- Galiński, M.; Lewandowski, A.; Stępniak, I. Ionic liquids as electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- König, A.; Weckesser, D.; Jensen, D. Ionische Flüssigkeiten – Analyse mittels Ionenchromatographie. GIT Labor-Fachzeitschrift 2006, 6, 546–549. [Google Scholar]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef]
- Holzapfel, M.; Jost, C.; Novak, P. Stable cycling of graphite in an ionic liquid based electrolyte. Chem. Commun. 2004, 2098–2099. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.E. What’s an ionic liquid? Interface 2007, 16, 38. [Google Scholar]
- Appetecchi, G.B.; Scaccia, S.; Tizzani, C.; Alessandrini, F.; Passerini, S. Synthesis of hydrophobic ionic liquids for electrochemical applications. J. Electrochem. Soc. 2006, 153, A1685–A1691. [Google Scholar] [CrossRef]
- Nakagawa, H.; Izuchi, S.; Kuwana, K.; Nukuda, T.; Aihara, Y. Liquid and Polymer Gel Electrolytes for Lithium Batteries Composed of Room-Temperature Molten Salt Doped by Lithium Salt. J. Electrochem. Soc. 2003, 150, A695–A700. [Google Scholar] [CrossRef]
- Klett, M.; Svens, P.; Tengstedt, C.; Seyeux, A.; Światowska, J.; Lindbergh, G.r.; Wreland Lindström, R. Uneven film formation across depth of porous graphite electrodes in cycled commercial Li-ion batteries. J. Phys. Chem. C 2014, 119, 90–100. [Google Scholar] [CrossRef]
- Börner, M.; Friesen, A.; Grützke, M.; Stenzel, Y.; Brunklaus, G.; Haetge, J.; Nowak, S.; Schappacher, F.; Winter, M. Correlation of aging and thermal stability of commercial 18650-type lithium ion batteries. J. Power Sources 2017, 342, 382–392. [Google Scholar] [CrossRef]
- Zhan, C.; Lu, J.; Jeremy Kropf, A.; Wu, T.; Jansen, A.N.; Sun, Y.K.; Qiu, X.; Amine, K. Mn(II) deposition on anodes and its effects on capacity fade in spinel lithium manganate-carbon systems. Nat. Commun. 2013, 4, 2437. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Ziv, B.; Luski, S.; Aurbach, D.; Halalay, I.C. Increasing the durability of Li-ion batteries by means of manganese ion trapping materials with nitrogen functionalities. J. Power Sources 2017, 341, 457–465. [Google Scholar] [CrossRef]
- Evertz, M.; Lürenbaum, C.; Vortmann, B.; Winter, M.; Nowak, S. Development of a method for direct elemental analysis of lithium ion battery degradation products by means of total reflection X-ray fluorescence. Spectrochim. Acta Part B 2015, 112, 34–39. [Google Scholar] [CrossRef]
- Evertz, M.; Horsthemke, F.; Kasnatscheew, J.; Börner, M.; Winter, M.; Nowak, S. Unraveling transition metal dissolution of Li1.04Ni1/3Co1/3Mn1/3O2 (NCM 111) in lithium ion full cells by using the total reflection X-ray fluorescence technique. J. Power Sources 2016, 329, 364–371. [Google Scholar] [CrossRef]
- Kasnatscheew, J.; Evertz, M.; Streipert, B.; Wagner, R.; Nowak, S.; Cekic Laskovic, I.; Winter, M. Changing established belief on capacity fade mechanisms: Thorough investigation of LiNi1/3Co1/3Mn1/3O2 (NCM111) under high voltage conditions. J. Phys. Chem. C 2017, 121, 1521–1529. [Google Scholar] [CrossRef]
- Börner, M.; Horsthemke, F.; Kollmer, F.; Haseloff, S.; Friesen, A.; Niehoff, P.; Nowak, S.; Winter, M.; Schappacher, F.M. Degradation effects on the surface of commercial LiNi0.5Co0.2Mn0.3O2 electrodes. J. Power Sources 2016, 335, 45–55. [Google Scholar] [CrossRef]
- Nowak, S.; Winter, M. Elemental analysis of lithium ion batteries. J. Anal. Atom. Spectrom. 2017, 32, 1833–1847. [Google Scholar] [CrossRef]
- Evertz, M.; Schwieters, T.; Börner, M.; Winter, M.; Nowak, S. Matrix-matched standards for the quantification of elemental lithium ion battery degradation products deposited on carbonaceous negative electrodes using pulsed-glow discharge-sector field-mass spectrometry. J. Anal. Atom. Spectrom. 2017, 32, 1862–1867. [Google Scholar] [CrossRef]
- Schwieters, T.; Evertz, M.; Fengler, A.; Börner, M.; Dagger, T.; Stenzel, Y.; Harte, P.; Winter, M.; Nowak, S. Visualizing elemental deposition patterns on carbonaceous anodes from lithium ion batteries: A laser ablation-inductively coupled plasma-mass spectrometry study on factors influencing the deposition of lithium, nickel, manganese and cobalt after dissolution and migration from the Li1[Ni1/3Mn1/3Co1/3]O2 and LiMn1.5 Ni0.5O4 cathode. J. Power Sources 2018, 380, 194–201. [Google Scholar]
- Harte, P.; Evertz, M.; Schwieters, T.; Diehl, M.; Winter, M.; Nowak, S. Adaptation and improvement of an elemental mapping method for lithium ion battery electrodes and separators by means of laser ablation-inductively coupled plasma-mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Terborg, L.; Nowak, S.; Passerini, S.; Winter, M.; Karst, U.; Haddad, P.R.; Nesterenko, P.N. Ion chromatographic determination of hydrolysis products of hexafluorophosphate salts in aqueous solution. Anal. Chim. Acta 2012, 714, 121–126. [Google Scholar] [CrossRef]
- Sloop, S.E.; Pugh, J.K.; Wang, S.; Kerr, J.; Kinoshita, K. Chemical Reactivity of PF 5 and LiPF6 in Ethylene Carbonate/Dimethyl Carbonate Solutions. Electrochem. Solid-State Lett. 2001, 4, A42–A44. [Google Scholar] [CrossRef]
- Sloop, S.E.; Kerr, J.B.; Kinoshita, K. The role of Li-ion battery electrolyte reactivity in performance decline and self-discharge. J. Power Sources 2003, 119, 330–337. [Google Scholar] [CrossRef]
- Blomgren, G.E. Electrolytes for advanced batteries. J. Power Sources 1999, 81, 112–118. [Google Scholar] [CrossRef]
- Kawamura, T.; Okada, S.; Yamaki, J.-i. Decomposition reaction of LiPF 6-based electrolytes for lithium ion cells. J. Power Sources 2006, 156, 547–554. [Google Scholar] [CrossRef]
- Plakhotnyk, A.V.; Ernst, L.; Schmutzler, R. Hydrolysis in the system LiPF 6—propylene carbonate—dimethyl carbonate—H 2 O. J. Fluorine Chem. 2005, 126, 27–31. [Google Scholar] [CrossRef]
- Nowak, S.; Winter, M. The role of sub-and supercritical CO2 as “Processing Solvent” for the recycling and sample preparation of lithium ion battery electrolytes. Molecules 2017, 22, 403. [Google Scholar] [CrossRef]
- Patnaik, P. A Comprehensive Guide to the Hazardous Properties of Chemical Substances; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Delfino, R.T.; Ribeiro, T.S.; Figueroa-Villar, J.D. Organophosphorus compounds as chemical warfare agents: A review. J. Brazil. Chem. Soc. 2009, 20, 407–428. [Google Scholar] [CrossRef]
- Vortmann-Westhoven, B.; Lürenbaum, C.; Winter, M.; Nowak, S. Determination of lithium and transition metals in Li1Ni1/3Co1/3Mn1/3O2 (NCM) cathode material for lithium-ion batteries by capillary electrophoresis. Electrophoresis 2017, 38, 540–546. [Google Scholar] [CrossRef]
- Vortmann-Westhoven, B.; Diehl, M.; Winter, M.; Nowak, S. Ion Chromatography with Post-column Reaction and Serial Conductivity and Spectrophotometric Detection Method Development for Quantification of Transition Metal Dissolution in Lithium Ion Battery Electrolytes. Chromatographia 2018, 1–8. [Google Scholar] [CrossRef]
- Hao, F.; Haddad, P.R.; Ruther, T. IC Determination of Halide Impurities in Ionic Liquids. Chromatographia 2008, 67, 495–498. [Google Scholar] [CrossRef]
- Villagrán, C.; Deetlefs, M.; Pitner, W.R.; Hardacre, C. Quantification of Halide in Ionic Liquids Using Ion Chromatography. Anal. Chem. 2004, 76, 2118–2123. [Google Scholar] [CrossRef]
- Zhou, S.; Yu, H.; Yang, L.; Ai, H. Fast determination of tetrafluoroborate by high-performance liquid chromatography using a monolithic column. J. Chromatogr. A 2008, 1206, 200–203. [Google Scholar] [CrossRef]
- Gao, W.; Yu, H.; Zhou, S. Determination of Imidazolium Ionic Liquid Cations by Ion-Pair Chromatography Using a Monolithic Column and Direct Conductivity Detection. Chromatographia 2010, 71, 475–479. [Google Scholar] [CrossRef]
- Meng, L.; Yu, H.; Liu, Y. Determination of Pyridinium Ionic Liquid Cations by Reversed Phase Ion-Pair Chromatography Using Gradient Elution. Chromatographia 2011, 73, 367–371. [Google Scholar] [CrossRef]
- Markuszewski, M.J.; Stepnowski, P.; Marszałł, M.P. Capillary electrophoretic separation of cationic constituents of imidazolium ionic liquids. Electrophoresis 2004, 25, 3450–3454. [Google Scholar] [CrossRef]
- Qin, W.; Wei, H.; Li, S.F.Y. Separation of ionic liquid cations and related imidazole derivatives by α-cyclodextrin modified capillary zone electrophoresis. Analyst 2002, 127, 490–493. [Google Scholar] [CrossRef]
- Markowska, A.; Stepnowski, P. Capillary isotachophoresis for the analysis of ionic liquid entities. J. Sep. Sci. 2010, 33, 1991–1996. [Google Scholar] [CrossRef]
- Kosobucki, P.; Buszewski, B. Isotachophoretic separation of selected imidazolium ionic liquids. Talanta 2008, 74, 1670–1674. [Google Scholar] [CrossRef]
- Pyschik, M.; Klein-Hitpaß, M.; Girod, S.; Winter, M.; Nowak, S. Capillary electrophoresis with contactless conductivity detection for the quantification of fluoride in lithium ion battery electrolytes and in ionic liquids—A comparison to the results gained with a fluoride ion-selective electrode. Electrophoresis 2017, 38, 533–539. [Google Scholar] [CrossRef]
- Wilken, A.; Kraft, V.; Girod, S.; Winter, M.; Nowak, S. A fluoride-selective electrode (Fse) for the quantification of fluoride in lithium-ion battery (Lib) electrolytes. Anal. Methods 2016, 8, 6932–6940. [Google Scholar] [CrossRef] [Green Version]
- Pyschik, M.; Kraft, V.; Passerini, S.; Winter, M.; Nowak, S. Thermal aging of anions in ionic liquids containing lithium salts by IC/ESI-MS. Electrochim. Acta 2014, 130, 426–430. [Google Scholar] [CrossRef]
- Pyschik, M.; Schultz, C.; Passerini, S.; Winter, M.; Nowak, S. Aging of cations of ionic liquids monitored by ion chromatography hyphenated to an electrospray ionization mass spectrometer. Electrochim. Acta 2015, 176, 1143–1152. [Google Scholar] [CrossRef]
- Pyschik, M.; Winter, M.; Nowak, S. Determination and quantification of cations in ionic liquids by capillary electrophoresis-mass spectrometry. J. Chromatogr. A 2017, 1485, 131–141. [Google Scholar] [CrossRef]
- Chancelier, L.; Diallo, A.; Santini, C.; Marlair, G.; Gutel, T.; Mailley, S.; Len, C. Targeting adequate thermal stability and fire safety in selecting ionic liquid-based electrolytes for energy storage. Phys. Chem. Chem. Phys. 2014, 16, 1967–1976. [Google Scholar] [CrossRef] [Green Version]
- Chancelier, L.; Benayad, A.; Gutel, T.; Mailley, S.; Santini, C.C. Characterisation of LTO//NMC Batteries Containing Ionic Liquids or Carbonates after Cycling and Overcharge. ECS Trans. 2014, 62, 235–246. [Google Scholar] [CrossRef]
- Chancelier, L.; Benayad, A.; Gutel, T.; Mailley, S.; Santini, C. Characterization of LTO//NMC Batteries Containing Ionic Liquid or Carbonate Electrolytes after Cycling and Overcharge. J. Electrochem. Soc. 2015, 162, A1008–A1013. [Google Scholar] [CrossRef] [Green Version]
- Gudzinowicz, B.; Driscoll, J. Separation and Analysis of Organic Carbonates by Gas Chromatography. Anal. Chem. 1961, 33, 1508–1510. [Google Scholar] [CrossRef]
- Yoza, N.; Nakashima, S.; Ueda, N.; Miyajima, T.; Nakamura, T.; Vast, P. High-performance liquid chromatographic characterization of monofluorophosphate, difluorophosphate and hexafluorophosphate. J. Chromatogr. A 1994, 664, 111–116. [Google Scholar] [CrossRef]
- Arakawa, M.; Yamaki, J.-i. Anodic oxidation of propylene carbonate and ethylene carbonate on graphite electrodes. J. Power Sources 1995, 54, 250–254. [Google Scholar] [CrossRef]
- Ohta, A.; Koshina, H.; Okuno, H.; Murai, H. Relationship between carbonaceous materials and electrolyte in secondary lithium-ion batteries. J. Power Sources 1995, 54, 6–10. [Google Scholar] [CrossRef]
- Kominato, A.; Yasukawa, E.; Sato, N.; Ijuuin, T.; Asahina, H.; Mori, S. Analysis of surface films on lithium in various organic electrolytes. J. Power Sources 1997, 68, 471–475. [Google Scholar] [CrossRef]
- Mori, S.; Asahina, H.; Suzuki, H.; Yonei, A.; Yokoto, K. Chemical properties of various organic electrolytes for lithium rechargeable batteries: 1. Characterization of passivating layer formed on graphite in alkyl carbonate solutions. J. Power Sources 1997, 68, 59–64. [Google Scholar] [CrossRef]
- Johnson, B.A.; White, R.E. Characterization of commercially available lithium-ion batteries. J. Power Sources 1998, 70, 48–54. [Google Scholar] [CrossRef]
- Heider, U.; Oesten, R.; Jungnitz, M. Challenge in manufacturing electrolyte solutions for lithium and lithium ion batteries quality control and minimizing contamination level. J. Power Sources 1999, 81, 119–122. [Google Scholar] [CrossRef]
- Kumai, K.; Miyashiro, H.; Kobayashi, Y.; Takei, K.; Ishikawa, R. Gas generation mechanism due to electrolyte decomposition in commercial lithium-ion cell. J. Power Sources 1999, 81, 715–719. [Google Scholar] [CrossRef]
- Gourdin, G.; Collins, J.; Zheng, D.; Foster, M.; Qu, D. Spectroscopic compositional analysis of electrolyte during initial SEI layer formation. J. Phys. Chem. C 2014, 118, 17383–17394. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Takeda, S.; Kaneko, I.; Yoshitake, H.; Yanagida, M.; Saito, Y.; Sakai, T. An approach of evaluating the effect of vinylene carbonate additive on graphite anode for lithium ion battery at elevated temperature. Electrochem. Commun. 2015, 61, 70–73. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Takeda, S.; Kaneko, I.; Yoshitake, H.; Yanagida, M.; Saito, Y.; Sakai, T. Formation of thermally resistant films induced by vinylene carbonate additive on a hard carbon anode for lithium ion batteries at elevated temperature. RSC Adv. 2016, 6, 75777–75781. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Takeda, S.; Kaneko, I.; Yoshitake, H.; Mukai, T.; Yanagida, M.; Saito, Y.; Sakai, T. Understanding the Improved High-Temperature Cycling Stability of a LiNi0. 5Mn0. 3Co0. 2O2/Graphite Cell with Vinylene Carbonate: A Comprehensive Analysis Approach Utilizing LC-MS and DART-MS. J. Phys. Chem. C 2018, 122, 5864–5870. [Google Scholar] [CrossRef]
- Tochihara, M.; Nara, H.; Mukoyama, D.; Yokoshima, T.; Momma, T.; Osaka, T. Liquid chromatography-quadruple time of flight mass spectrometry analysis of products in degraded lithium-ion batteries. J. Electrochem. Soc. 2015, 162, A2008–A2015. [Google Scholar] [CrossRef]
- Takeda, S.; Morimura, W.; Liu, Y.H.; Sakai, T.; Saito, Y. Identification and formation mechanism of individual degradation products in lithium-ion batteries studied by liquid chromatography/electrospray ionization mass spectrometry and atmospheric solid analysis probe mass spectrometry. Rapid Commun. Mass Spectrom. 2016, 30, 1754–1762. [Google Scholar] [CrossRef] [PubMed]
- Kraft, V.; Weber, W.; Streipert, B.; Wagner, R.; Schultz, C.; Winter, M.; Nowak, S. Qualitative and quantitative investigation of organophosphates in an electrochemically and thermally treated lithium hexafluorophosphate-based lithium ion battery electrolyte by a developed liquid chromatography-tandem quadrupole mass spectrometry method. RSC Adv. 2016, 6, 8–17. [Google Scholar] [CrossRef] [Green Version]
- Schultz, C.; Vedder, S.; Streipert, B.; Winter, M.; Nowak, S. Quantitative investigation of the decomposition of organic lithium ion battery electrolytes with LC-MS/MS. RSC Adv. 2017, 7, 27853–27862. [Google Scholar] [CrossRef] [Green Version]
- Henschel, J.; Winter, M.; Nowak, S.; Jiang, W. Analysis of organophosphates in lithium ion battery electrolytes by HILIC-ESI-MS. LC GC 2018, 30, 691. [Google Scholar]
- Henschel, J.; Schwarz, L.; Glorius, F.; Winter, M.; Nowak, S. Further insights into structural diversity of phosphorus-based decomposition products in lithium ion battery electrolytes via liquid chromatographic techniques hyphenated to ion trap - time of flight mass spectrometry. Anal. Chem. 2019, 91, 3980–3988. [Google Scholar] [CrossRef]
- Bloom, I.; Bareño, J.; Dietz Rago, N.; Dogan, F.; Graczyk, D.G.; Tsai, Y.; Naik, S.R.; Han, S.-D.; Lee, E.; Du, Z.; et al. Effect of overcharge on Li(Ni0.5Mn0.3Co0.2)O2 cathodes: NMP-soluble binder. II—Chemical changes in the anode. J. Power Sources 2018, 385, 156–164. [Google Scholar] [CrossRef]
- Tasaki, K.; Kanda, K.; Nakamura, S.; Ue, M. Decomposition of LiPF6and Stability of PF 5 in Li-Ion Battery Electrolytes Density Functional Theory and Molecular Dynamics Studies. J. Electrochem. Soc. 2003, 150, A1628–A1636. [Google Scholar] [CrossRef]
- Terborg, L.; Weber, S.; Blaske, F.; Passerini, S.; Winter, M.; Karst, U.; Nowak, S. Investigation of thermal aging and hydrolysis mechanisms in commercial lithium ion battery electrolyte. J. Power Sources 2013, 242, 832–837. [Google Scholar] [CrossRef]
- Kraft, V.; Weber, W.; Grützke, M.; Winter, M.; Nowak, S. Study of decomposition products by gas chromatography-mass spectrometry and ion chromatography-electrospray ionization-mass spectrometry in thermally decomposed lithium hexafluorophosphate-based lithium ion battery electrolytes. RSC Adv. 2015, 5, 80150–80157. [Google Scholar] [CrossRef] [Green Version]
- Handel, P.; Fauler, G.; Kapper, K.; Schmuck, M.; Stangl, C.; Fischer, R.; Uhlig, F.; Koller, S. Thermal aging of electrolytes used in lithium-ion batteries–An investigation of the impact of protic impurities and different housing materials. J. Power Sources 2014, 267, 255–259. [Google Scholar] [CrossRef]
- Menzel, J.; Schultz, H.; Kraft, V.; Badillo, J.P.; Winter, M.; Nowak, S. Quantification of ionic organo (fluoro) phosphates in decomposed lithium battery electrolytes. RSC Adv. 2017, 7, 39314–39324. [Google Scholar] [CrossRef]
- Stich, M.; Göttlinger, M.; Kurniawan, M.; Schmidt, U.; Bund, A. Hydrolysis of LiPF6 in Carbonate-Based Electrolytes for Lithium-Ion Batteries and in Aqueous Media. J. Phys. Chem. C 2018, 122, 8836–8842. [Google Scholar] [CrossRef]
- Ota, H.; Sato, T.; Suzuki, H.; Usami, T. TPD–GC/MS analysis of the solid electrolyte interface (SEI) on a graphite anode in the propylene carbonate/ethylene sulfite electrolyte system for lithium batteries. J. Power Sources 2001, 97, 107–113. [Google Scholar] [CrossRef]
- Sano, A.; Kurihara, M.; Ogawa, K.; Iijima, T.; Maruyama, S. Decreasing the initial irreversible capacity loss of graphite negative electrode by alkali-addition. J. Power Sources 2009, 192, 703–707. [Google Scholar] [CrossRef]
- Ogumi, Z.; Sano, A.; Inaba, M.; Abe, T. Pyrolysis/gas chromatography/mass spectroscopy analysis of the surface film formed on graphite negative electrode. J. Power Sources 2001, 97, 156–158. [Google Scholar] [CrossRef]
- Mogi, R.; Inaba, M.; Iriyama, Y.; Abe, T.; Ogumi, Z. Study of the Decomposition of Propylene Carbonate on Lithium Metal Surface by Pyrolysis− Gas Chromatography− Mass Spectroscopy. Langmuir 2003, 19, 814–821. [Google Scholar] [CrossRef]
- Zhang, X.; Ross, P.; Kostecki, R.; Kong, F.; Sloop, S.; Kerr, J.; Striebel, K.; Cairns, E.; McLarnon, F. Diagnostic characterization of high power lithium-ion batteries for use in hybrid electric vehicles. J. Electrochem. Soc. 2001, 148, A463–A470. [Google Scholar] [CrossRef]
- Aurbach, D.; Markovsky, B.; Rodkin, A.; Cojocaru, M.; Levi, E.; Kim, H.-J. An analysis of rechargeable lithium-ion batteries after prolonged cycling. Electrochim. Acta 2002, 47, 1899–1911. [Google Scholar] [CrossRef]
- Gireaud, L.; Grugeon, S.; Pilard, S.; Guenot, P.; Tarascon, J.-M.; Laruelle, S. Mass spectrometry investigations on electrolyte degradation products for the development of nanocomposite electrodes in lithium ion batteries. Anal. Chem. 2006, 78, 3688–3698. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lucht, B.L. Lithium-ion batteries: Thermal reactions of electrolyte with the surface of metal oxide cathode particles. J. Electrochem. Soc. 2006, 153, A1617–A1625. [Google Scholar] [CrossRef]
- Li, W.; Lucht, B.L. Inhibition of solid electrolyte interface formation on cathode particles for lithium-ion batteries. J. Power Sources 2007, 168, 258–264. [Google Scholar] [CrossRef]
- Xiao, A.; Li, W.; Lucht, B.L. Thermal reactions of mesocarbon microbead (MCMB) particles in LiPF6-based electrolyte. J. Power Sources 2006, 162, 1282–1288. [Google Scholar] [CrossRef]
- Gachot, G.; Grugeon, S.; Armand, M.; Pilard, S.; Guenot, P.; Tarascon, J.-M.; Laruelle, S. Deciphering the multi-step degradation mechanisms of carbonate-based electrolyte in Li batteries. J. Power Sources 2008, 178, 409–421. [Google Scholar] [CrossRef]
- Yang, L.; Smith, C.; Patrissi, C.; Schumacher, C.R.; Lucht, B.L. Surface reactions and performance of non-aqueous electrolytes with lithium metal anodes. J. Power Sources 2008, 185, 1359–1366. [Google Scholar] [CrossRef]
- Eom, J.-Y.; Jung, I.-H.; Lee, J.-H. Effects of vinylene carbonate on high temperature storage of high voltage Li-ion batteries. J. Power Sources 2011, 196, 9810–9814. [Google Scholar] [CrossRef]
- Petibon, R.; Rotermund, L.; Nelson, K.; Gozdz, A.; Xia, J.; Dahn, J. Study of electrolyte components in Li ion cells using liquid-liquid extraction and gas chromatography coupled with mass spectrometry. J. Electrochem. Soc. 2014, 161, A1167–A1172. [Google Scholar] [CrossRef]
- Petibon, R.; Xia, J.; Burns, J.; Dahn, J. Study of the Consumption of Vinylene Carbonate in Li [Ni0. 33Mn0. 33Co0. 33] O2/Graphite Pouch Cells. J. Electrochem. Soc. 2014, 161, A1618–A1624. [Google Scholar] [CrossRef]
- Petibon, R.; Rotermund, L.; Dahn, J. Evaluation of phenyl carbonates as electrolyte additives in lithium-ion batteries. J. Power Sources 2015, 287, 184–195. [Google Scholar] [CrossRef]
- Petibon, R.; Chevrier, V.; Aiken, C.; Hall, D.; Hyatt, S.; Shunmugasundaram, R.; Dahn, J. Studies of the capacity fade mechanisms of LiCoO2/Si-alloy: Graphite cells. J. Electrochem. Soc. 2016, 163, A1146–A1156. [Google Scholar] [CrossRef]
- Gachot, G.; Ribière, P.; Mathiron, D.; Grugeon, S.; Armand, M.; Leriche, J.-B.; Pilard, S.; Laruelle, S. Gas chromatography/mass spectrometry as a suitable tool for the Li-ion battery electrolyte degradation mechanisms study. Anal. Chem. 2011, 83, 478–485. [Google Scholar] [CrossRef]
- Gachot, G.; Grugeon, S.; Jimenez-Gordon, I.; Eshetu, G.G.; Boyanov, S.; Lecocq, A.; Marlair, G.; Pilard, S.; Laruelle, S. Gas chromatography/Fourier transform infrared/mass spectrometry coupling: A tool for Li-ion battery safety field investigation. Anal. Methods 2014, 6, 6120–6124. [Google Scholar] [CrossRef]
- Kim, H.; Grugeon, S.; Gachot, G.; Armand, M.; Sannier, L.; Laruelle, S. Ethylene bis-carbonates as telltales of SEI and electrolyte health, role of carbonate type and new additives. Electrochim. Acta 2014, 136, 157–165. [Google Scholar] [CrossRef]
- Terborg, L.; Weber, S.; Passerini, S.; Winter, M.; Karst, U.; Nowak, S. Development of gas chromatographic methods for the analyses of organic carbonate-based electrolytes. J. Power Sources 2014, 245, 836–840. [Google Scholar] [CrossRef]
- Grützke, M.; Kraft, V.; Weber, W.; Wendt, C.; Friesen, A.; Klamor, S.; Winter, M.; Nowak, S. Supercritical carbon dioxide extraction of lithium-ion battery electrolytes. J. Supercrit. Fluid. 2014, 94, 216–222. [Google Scholar] [CrossRef]
- Grützke, M.; Mönnighoff, X.; Horsthemke, F.; Kraft, V.; Winter, M.; Nowak, S. Extraction of lithium-ion battery electrolytes with liquid and supercritical carbon dioxide and additional solvents. RSC Adv. 2015, 5, 43209–43217. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Mu, D.; Zheng, R.; Dai, C. Supercritical CO 2 extraction of organic carbonate-based electrolytes of lithium-ion batteries. RSC Adv. 2014, 4, 54525–54531. [Google Scholar] [CrossRef]
- Liu, Y.; Mu, D.; Dai, Y.; Ma, Q.; Zheng, R.; Dai, C. Analysis on Extraction Behaviour of Lithium-ion Battery Electrolyte Solvents in Supercritical CO2 by Gas Chromatography. Int. J. Electrochem. Sci. 2016, 11, 7594–7604. [Google Scholar] [CrossRef]
- Mönnighoff, X.; Friesen, A.; Konersmann, B.; Horsthemke, F.; Grützke, M.; Winter, M.; Nowak, S. Supercritical carbon dioxide extraction of electrolyte from spent lithium ion batteries and its characterization by gas chromatography with chemical ionization. J. Power Sources 2017, 352, 56–63. [Google Scholar] [CrossRef]
- Horsthemke, F.; Winkler, V.; Diehl, M.; Winter, M.; Nowak, S. Concept of the Analysis of the Electrolyte Composition Within the Cell Manufacturing Process: From Sealing to Sample Preparation. Energy Technol. 2019. [Google Scholar] [CrossRef]
- Ortiz, D.; Steinmetz, V.; Durand, D.; Legand, S.; Dauvois, V.; Maître, P.; Le Caër, S. Radiolysis as a solution for accelerated ageing studies of electrolytes in Lithium-ion batteries. Nat. Commun. 2015, 6, 6950. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, D.; Jimenez Gordon, I.; Legand, S.; Dauvois, V.; Baltaze, J.-P.; Marignier, J.-L.; Martin, J.-F.; Belloni, J.; Mostafavi, M.; Le Caër, S. Role of PF6− in the radiolytical and electrochemical degradation of propylene carbonate solutions. J. Power Sources 2016, 326, 285–295. [Google Scholar] [CrossRef]
- Weber, W.; Kraft, V.; Grützke, M.; Wagner, R.; Winter, M.; Nowak, S. Identification of alkylated phosphates by gas chromatography–mass spectrometric investigations with different ionization principles of a thermally aged commercial lithium ion battery electrolyte. J. Chromatogr. A 2015, 1394, 128–136. [Google Scholar] [CrossRef]
- Grützke, M.; Krüger, S.; Kraft, V.; Vortmann, B.; Rothermel, S.; Winter, M.; Nowak, S. Investigation of the Storage Behavior of Shredded Lithium-Ion Batteries from Electric Vehicles for Recycling Purposes. ChemSusChem 2015, 8, 3433–3438. [Google Scholar] [CrossRef]
- Michan, A.L.; Parimalam, B.S.; Leskes, M.; Kerber, R.N.; Yoon, T.; Grey, C.P.; Lucht, B.L. Fluoroethylene carbonate and vinylene carbonate reduction: Understanding lithium-ion battery electrolyte additives and solid electrolyte interphase formation. Chem. Mater. 2016, 28, 8149–8159. [Google Scholar] [CrossRef]
- Horsthemke, F.; Friesen, A.; Mönnighoff, X.; Stenzel, Y.P.; Grützke, M.; Andersson, J.T.; Winter, M.; Nowak, S. Fast screening method to characterize lithium ion battery electrolytes by means of solid phase microextraction–gas chromatography–mass spectrometry. RSC Adv. 2017, 7, 46989–46998. [Google Scholar] [CrossRef]
- Horsthemke, F.; Friesen, A.; Ibing, L.; Klein, S.; Winter, M.; Nowak, S. Possible carbon-carbon bond formation during decomposition? Characterization and identification of new decomposition products in lithium ion battery electrolytes by means of SPME-GC-MS. Electrochim. Acta 2019, 295, 401–409. [Google Scholar] [CrossRef]
- Strehlau, J.; Weber, T.; Lürenbaum, C.; Bornhorst, J.; Galla, H.-J.; Schwerdtle, T.; Winter, M.; Nowak, S. Towards quantification of toxicity of lithium ion battery electrolytes-development and validation of a liquid-liquid extraction GC-MS method for the determination of organic carbonates in cell culture materials. Anal. Bioanal. Chem. 2017, 409, 6123–6131. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, Y.P.; Winter, M.; Nowak, S. Evaluation of different plasma conditions and resolutions for understanding elemental organophosphorus analysis via GC-ICP-SF-MS. J. Anal. Atom. Spectrom. 2018, 33, 1041–1048. [Google Scholar] [CrossRef]
- Stenzel, Y.P.; Wiemers-Meyer, S.; Edel, J.; Winter, M.; Nowak, S. Analysis of acidic organo(fluoro)phosphates as decomposition product of lithium ion battery electrolytes via derivatization Gas Chromatography-Mass Spectrometry. J. Chromatogr. A 2019, 1592, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Smuts, J.; Walsh, P.; Fan, H.; Hildenbrand, Z.; Wong, D.; Wetz, D.; Schug, K.A. Permanent gas analysis using gas chromatography with vacuum ultraviolet detection. J. Chromatogr. A 2015, 1388, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Self, J.; Aiken, C.; Petibon, R.; Dahn, J. Survey of gas expansion in Li-ion NMC pouch cells. J. Electrochem. Soc. 2015, 162, A796–A802. [Google Scholar] [CrossRef]
- Wagner, M.R.; Raimann, P.; Trifonova, A.; Moeller, K.-C.; Besenhard, J.; Winter, M. Electrolyte decomposition reactions on tin-and graphite-based anodes are different. Electrochem. Solid-State Lett. 2004, 7, A201–A205. [Google Scholar] [CrossRef]
- Imhof, R.; Novák, P. In situ investigation of the electrochemical reduction of carbonate electrolyte solutions at graphite electrodes. J. Electrochem. Soc. 1998, 145, 1081–1087. [Google Scholar] [CrossRef]
- Wagner, M.R.; Raimann, P.; Trifonova, A.; Möller, K.-C.; Besenhard, J.; Winter, M. Dilatometric and mass spectrometric investigations on lithium ion battery anode materials. Anal. Bioanal. Chem. 2004, 379, 272–276. [Google Scholar] [PubMed]
- Tsiouvaras, N.; Meini, S.; Buchberger, I.; Gasteiger, H. A novel on-line mass spectrometer design for the study of multiple charging cycles of a Li-O2 battery. J. Electrochem. Soc. 2013, 160, A471–A477. [Google Scholar] [CrossRef]
- Metzger, M.; Strehle, B.; Solchenbach, S.; Gasteiger, H.A. Origin of H2 evolution in LIBs: H2O reduction vs. electrolyte oxidation. J. Electrochem. Soc. 2016, 163, A798–A809. [Google Scholar] [CrossRef]
- Onuki, M.; Kinoshita, S.; Sakata, Y.; Yanagidate, M.; Otake, Y.; Ue, M.; Deguchi, M. Identification of the Source of Evolved Gas in Li-Ion Batteries Using# 2# 1-labeled Solvents. J. Electrochem. Soc. 2008, 155, A794–A797. [Google Scholar]
- Yang, C.; Wang, Y.; Wan, C. Composition analysis of the passive film on the carbon electrode of a lithium-ion battery with an EC-based electrolyte. J. Power Sources 1998, 72, 66–70. [Google Scholar] [CrossRef]
- Madec, L.; Petibon, R.; Tasaki, K.; Xia, J.; Sun, J.-P.; Hill, I.; Dahn, J. Mechanism of action of ethylene sulfite and vinylene carbonate electrolyte additives in LiNi 1/3 Mn 1/3 Co 1/3 O 2/graphite pouch cells: Electrochemical, GC-MS and XPS analysis. Phys. Chem. Chem. Phys. 2015, 17, 27062–27076. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.; Allen, J.; Thompson, L.; Harlow, J.; Stone, W.; Hill, I.; Dahn, J. Quantifying, Understanding and Evaluating the Effects of Gas Consumption in Lithium-Ion Cells. J. Electrochem. Soc. 2017, 164, A3518–A3528. [Google Scholar] [CrossRef] [Green Version]
- Ota, H.; Sakata, Y.; Inoue, A.; Yamaguchi, S. Analysis of vinylene carbonate derived SEI layers on graphite anode. J. Electrochem. Soc. 2004, 151, A1659–A1669. [Google Scholar] [CrossRef]
- Shin, J.-S.; Han, C.-H.; Jung, U.-H.; Lee, S.-I.; Kim, H.-J.; Kim, K. Effect of Li2CO3 additive on gas generation in lithium-ion batteries. J. Power Sources 2002, 109, 47–52. [Google Scholar] [CrossRef]
- Matasso, A.; Wong, D.; Wetz, D.; Liu, F. Effects of high-rate cycling on the bulk internal pressure rise and capacity degradation of commercial LiCoO2 cells. J. Electrochem. Soc. 2015, 162, A885–A891. [Google Scholar] [CrossRef]
- Wu, K.; Yang, J.; Zhang, Y.; Wang, C.; Wang, D. Investigation on Li4Ti5O12 batteries developed for hybrid electric vehicle. J. Appl. Electrochem. 2012, 42, 989–995. [Google Scholar] [CrossRef]
- Kong, W.; Li, H.; Huang, X.; Chen, L. Gas evolution behaviors for several cathode materials in lithium-ion batteries. J. Power Sources 2005, 142, 285–291. [Google Scholar] [CrossRef]
- Ohsaki, T.; Kishi, T.; Kuboki, T.; Takami, N.; Shimura, N.; Sato, Y.; Sekino, M.; Satoh, A. Overcharge reaction of lithium-ion batteries. J. Power Sources 2005, 146, 97–100. [Google Scholar] [CrossRef]
- Lamb, J.; Orendorff, C.J.; Roth, E.P.; Langendorf, J. Studies on the Thermal Breakdown of Common Li-Ion Battery Electrolyte Components. J. Electrochem. Soc. 2015, 162, A2131–A2135. [Google Scholar] [CrossRef]
- Abraham, D.; Roth, E.; Kostecki, R.; McCarthy, K.; MacLaren, S.; Doughty, D. Diagnostic examination of thermally abused high-power lithium-ion cells. J. Power Sources 2006, 161, 648–657. [Google Scholar] [CrossRef]
- Golubkov, A.W.; Fuchs, D.; Wagner, J.; Wiltsche, H.; Stangl, C.; Fauler, G.; Voitic, G.; Thaler, A.; Hacker, V. Thermal-runaway experiments on consumer Li-ion batteries with metal-oxide and olivin-type cathodes. RSC Adv. 2014, 4, 3633–3642. [Google Scholar] [CrossRef] [Green Version]
- Golubkov, A.W.; Scheikl, S.; Planteu, R.; Voitic, G.; Wiltsche, H.; Stangl, C.; Fauler, G.; Thaler, A.; Hacker, V. Thermal runaway of commercial 18650 Li-ion batteries with LFP and NCA cathodes–impact of state of charge and overcharge. RSC Adv. 2015, 5, 57171–57186. [Google Scholar] [CrossRef]
- Lammer, M.; Königseder, A.; Hacker, V. Holistic methodology for characterisation of the thermally induced failure of commercially available 18650 lithium ion cells. RSC Adv. 2017, 7, 24425–24429. [Google Scholar] [CrossRef]
- Lammer, M.; Königseder, A.; Gluschitz, P.; Hacker, V. Influence of aging on the heat and gas emissions from commercial lithium ion cells in case of thermal failure. J. Electrochem. Sci. Eng. 2018, 8, 101–110. [Google Scholar] [CrossRef]
- Koch, S.; Fill, A.; Birke, K.P. Comprehensive gas analysis on large scale automotive lithium-ion cells in thermal runaway. J. Power Sources 2018, 398, 106–112. [Google Scholar] [CrossRef]
- Doughty, D.H.; Roth, E.P.; Crafts, C.C.; Nagasubramanian, G.; Henriksen, G.; Amine, K. Effects of additives on thermal stability of Li ion cells. J. Power Sources 2005, 146, 116–120. [Google Scholar] [CrossRef]
- Roth, E.P. Abuse response of 18650 Li-ion cells with different cathodes using EC: EMC/LiPF6 and EC: PC: DMC/LiPF6 electrolytes. ECS Trans. 2008, 11, 19–41. [Google Scholar]
- Eshetu, G.G.; Grugeon, S.; Gachot, G.; Mathiron, D.; Armand, M.; Laruelle, S. LiFSI vs. LiPF6 electrolytes in contact with lithiated graphite: Comparing thermal stabilities and identification of specific SEI-reinforcing additives. Electrochim. Acta 2013, 102, 133–141. [Google Scholar] [CrossRef]
- Dugas, R.; Ponrouch, A.; Gachot, G.; David, R.; Palacín, M.R.; Tarascon, J.-M. Na reactivity toward carbonate-based electrolytes: The effect of FEC as additive. J. Electrochem. Soc. 2016, 163, A2333–A2339. [Google Scholar] [CrossRef]
- Roth, E.P.; Orendorff, C.J. How electrolytes influence battery safety. Electrochem. Soc. Interf. 2012, 21, 45–49. [Google Scholar] [CrossRef]
- Seo, D.M.; Chalasani, D.; Parimalam, B.S.; Kadam, R.; Nie, M.; Lucht, B.L. Reduction reactions of carbonate solvents for lithium ion batteries. ECS Electrochem. Lett. 2014, 3, A91–A93. [Google Scholar] [CrossRef]
- Xiong, D.; Petibon, R.; Nie, M.; Ma, L.; Xia, J.; Dahn, J.R. Interactions between positive and negative electrodes in Li-Ion cells operated at high temperature and high voltage. J. Electrochem. Soc. 2016, 163, A546–A551. [Google Scholar] [CrossRef]
- Xiong, D.; Ellis, L.; Nelson, K.; Hynes, T.; Petibon, R.; Dahn, J. Rapid Impedance Growth and Gas Production at the Li-Ion Cell Positive Electrode in the Absence of a Negative Electrode. J. Electrochem. Soc. 2016, 163, A3069–A3077. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Varenne, F.; Ortiz, D.; Pinzio, V.; Mostafavi, M.; Le Caer, S. Degradation mechanisms of the ethylene carbonate/diethyl carbonate mixture studied by radiolysis. ChemPhysChem 2017, 18, 2799–2806. [Google Scholar] [CrossRef]
- Varenne, F.; Alper, J.; Miserque, F.; Bongu, C.S.; Boulineau, A.; Martin, J.-F.; Dauvois, V.; Demarque, A.; Bouhier, M.; Boismain, F. Ex situ solid electrolyte interphase synthesis via radiolysis of Li-ion battery anode-electrolyte system for improved coulombic efficiency. Sustain. Energy Fuels 2018, 2, 2100–2108. [Google Scholar] [CrossRef]
- Lee, K.H.; Song, E.H.; Lee, J.Y.; Jung, B.H.; Lim, H.S. Mechanism of gas build-up in a Li-ion cell at elevated temperature. J. Power Sources 2004, 132, 201–205. [Google Scholar] [CrossRef]
- Wu, K.; Yang, J.; Liu, Y.; Zhang, Y.; Wang, C.; Xu, J.; Ning, F.; Wang, D. Investigation on gas generation of Li4Ti5O12/LiNi1/3Co1/3Mn1/3O2 cells at elevated temperature. J. Power Sources 2013, 237, 285–290. [Google Scholar] [CrossRef]
- Liu, M.; He, Y.-B.; Lv, W.; Zhang, C.; Du, H.; Li, B.; Yang, Q.-H.; Kang, F. High catalytic activity of anatase titanium dioxide for decomposition of electrolyte solution in lithium ion battery. J. Power Sources 2014, 268, 882–886. [Google Scholar] [CrossRef]
- Korepp, C.; Kern, W.; Lanzer, E.; Raimann, P.; Besenhard, J.; Yang, M.; Möller, K.-C.; Shieh, D.-T.; Winter, M. 4-Bromobenzyl isocyanate versus benzyl isocyanate—New film-forming electrolyte additives and overcharge protection additives for lithium ion batteries. J. Power Sources 2007, 174, 637–642. [Google Scholar] [CrossRef]
- Waldmann, T.; Iturrondobeitia, A.; Kasper, M.; Ghanbari, N.; Aguesse, F.; Bekaert, E.; Daniel, L.; Genies, S.; Gordon, I.J.; Löble, M.W. Post-mortem analysis of aged lithium-ion batteries: Disassembly methodology and physico-chemical analysis techniques. J. Electrochem. Soc. 2016, 163, A2149–A2164. [Google Scholar] [CrossRef]
- Rosenberg, E.; Kanakaki, C.; Amon, A.; Gocheva, I.; Trifonova, A. Understanding the degradation processes of the electrolyte of lithium ion batteries by chromatographic analysis. Bulg. Chem. Commun. 2017, 49, 242–253. [Google Scholar]
















| Origin of the Gases | Detector | References |
|---|---|---|
| Formation | AED | [224] |
| MS | [195,218,225,226] | |
| TCD | [57,196,227] | |
| n/a | [228] | |
| Cyclic Aging | FID | [229] |
| MS | [230] | |
| TCD | [229] | |
| n/a | [231] | |
| Overcharge | FID | [152] |
| FTIR | [152,153] | |
| MS | [232] | |
| TCD | [153] | |
| n/a | [233] | |
| Thermal Runaway/Abuse Conditions (ARC, etc.) | FID | [234] |
| FTIR | [198] | |
| MS | [198,235] | |
| TCD | [234,236,237,238,239,240] | |
| VUV | [217] | |
| n/a | [241,242] | |
| Model Aging of LIB Components (Thermal; Radiolysis, etc.) | FTIR | [243,244] |
| MS | [151,207,208,211,243,244,245,246,247,248,249,250] | |
| TCD | [207,208,249,250] | |
| n/a | [251,252,253] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stenzel, Y.P.; Horsthemke, F.; Winter, M.; Nowak, S. Chromatographic Techniques in the Research Area of Lithium Ion Batteries: Current State-of-the-Art. Separations 2019, 6, 26. https://0-doi-org.brum.beds.ac.uk/10.3390/separations6020026
Stenzel YP, Horsthemke F, Winter M, Nowak S. Chromatographic Techniques in the Research Area of Lithium Ion Batteries: Current State-of-the-Art. Separations. 2019; 6(2):26. https://0-doi-org.brum.beds.ac.uk/10.3390/separations6020026
Chicago/Turabian StyleStenzel, Yannick Philipp, Fabian Horsthemke, Martin Winter, and Sascha Nowak. 2019. "Chromatographic Techniques in the Research Area of Lithium Ion Batteries: Current State-of-the-Art" Separations 6, no. 2: 26. https://0-doi-org.brum.beds.ac.uk/10.3390/separations6020026