Solid-Phase Extraction of Aristolochic Acid I from Natural Plant Using Dual Ionic Liquid-Immobilized ZIF-67 as Sorbent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Apparatus
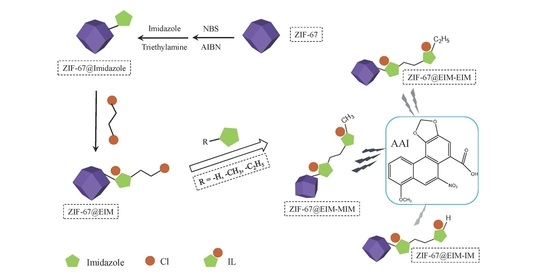
2.3. Preparation of ZIF-67, ZIF-67@Br and ZIF-67@Imidazole
2.4. Synthesis of the Three ZIF-67@EIM-ILs
2.5. Adsorption Isothermal and Kinetics Studies
2.6. Solid-Phase Extraction of AAI from Fibraurea Recisa Pierre
3. Results and Discussions
3.1. Characterization
3.2. Adsorption Isothermal and Kinetics Studies on the Three ZIF-67@EIM-ILs
3.3. Solid-Phase Extraction of AAI from Real Sample
3.4. Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, C.; Chang, W.; Lee, J.; Chang, T.; Huang, Y.; Hirasaki, Y.; Chen, H.; Imai, K.; Chen, S. Proteomics analysis of altered proteins in kidney of mice with aristolochic acid nephropathy using the fluorogenic derivatization-liquid chromatography-tandem mass spectrometry method. Biomed. Chromatogr. 2018, 32, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Sun, W.; Wang, B.; Zhao, H.; Li, Y.; Cai, S.; Xiang, L.; Zhu, Y.; Yao, H.; Song, J.; et al. An integrated system for identifying the hidden assassins in traditional medicines containing aristolochic acids. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Tian, M.; Row, K.H.; Yan, X.; Xiao, W. Isolation of aristolochic acid I from herbal plant using molecular imprinted polymer composited ionic liquid-based zeolitic imidazolate framework-67. J. Sep. Sci. 2019, 42, 3047–3053. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Serpa, A.; Pacheco-Fernández, I.; Pasán, J.; Pino, V. Metal–organic frameworks as key materials for solid-phase microextraction devices—A review. Separations 2019, 6, 47. [Google Scholar] [CrossRef] [Green Version]
- Rocío-Bautista, P.; Taima-Mancera, I.; Pasán, J.; Pino, V. Metal-organic frameworks in green analytical chemistry. Separations 2019, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Zhong, G.; Liu, D.; Zhang, J. The application of ZIF-67 and its derivatives: Adsorption, separation, electrochemistry and catalysts. J. Mater. Chem. A 2018, 6, 1887–1899. [Google Scholar] [CrossRef]
- Phuoc, N.M.; Jung, E.; Tran, N.A.T.; Lee, Y.; Yoo, C.; Kang, B.; Cho, Y. Enhanced desalination performance of capacitive deionization using nanoporous carbon derived from ZIF-67 metal organic frameworks and CNTs. Nanomaterials 2020, 10, 2091. [Google Scholar] [CrossRef]
- Tang, J.; Jiang, S.; Liu, Y.; Zheng, S.; Bai, L.; Guo, J.; Wang, J. Electrochemical determination of dopamine and uric acid using a glassy carbon electrode modified with a composite consisting of a Co(II)-based metalorganic framework (ZIF-67) and graphene oxide. Microchim. Acta 2018, 185, 1–11. [Google Scholar] [CrossRef]
- Haldorai, Y.; Choe, S.R.; Huh, Y.S.; Han, Y.K. A composite consisting of microporous carbon and cobalt(III) oxide and prepared from zeolitic imidazolate framework-67 for voltammetric determination of ascorbic acid. Microchim. Acta 2018, 185, 1–10. [Google Scholar] [CrossRef]
- Li, Q.; Guo, J.; Zhu, H.; Yan, F. Space-confined synthesis of ZIF- 67 nanoparticles in hollow carbon nanospheres for CO2 adsorption. Small 2019, 15, 1–8. [Google Scholar] [CrossRef]
- Lin, K.Y.A.; Chang, H.A. Ultra-high adsorption capacity of zeolitic imidazole framework-67 (ZIF-67) for removal of malachite green from water. Chemosphere 2015, 139, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Lu, R.; Ren, S.; Zhou, H.; Wu, Q.; Zhen, Y.; Chen, Z.; Fang, S. Magnetic beads embedded in poly(sodium-p-styrenesulfonate) and ZIF-67: Removal of nitrophenol from water. J. Solid State Chem. 2018, 265, 200–207. [Google Scholar] [CrossRef]
- Yang, Q.; Ren, S.; Zhao, Q.; Lu, R.; Hang, C.; Chen, Z.; Zheng, H. Selective separation of methyl orange from water using magnetic ZIF-67 composites. Chem. Eng. J. 2018, 333, 49–57. [Google Scholar] [CrossRef]
- Yang, Q.; Lu, R.; Ren, S.; Chen, C.; Chen, Z.; Yang, X. Three dimensional reduced graphene oxide/ZIF-67 aerogel: Effective removal cationic and anionic dyes from water. Chem. Eng. J. 2018, 348, 202–211. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, D.; Yu, H.; Jiang, S.; Zhang, S.; Zhang, X. Study on improving the stability of adsorption-encapsulation immobilized Laccase@ZIF-67. Biotechnol. Rep. 2020, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Zhang, X.; Feng, P.; Chai, H.; Huang, Y. ZIF-67 derived hollow cobalt sulfide as superior adsorbent for effective adsorption removal of ciprofloxacin antibiotics. Chem. Eng. J. 2018, 344, 95–104. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, X.; Hua, X.; Feng, R.; Zhou, M.; Cui, W. Development of zeolitic imidazolate framework-67 functionalized Co-Al LDH for CO2 adsorption. Colloids Surf. 2018, 552, 16–23. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Zhao, L. Magnetic nanocomposites derived from hollow ZIF-67 and core-shell ZIF-67@ZIF-8: Synthesis, properties, and adsorption of Rhodamine B. Eur. J. Inorg. Chem. 2017, 2017, 4110–4116. [Google Scholar] [CrossRef]
- Knebel, A.; Wulfert-Holzmann, P.; Friebe, S.; Pavel, J.; Strauß, I.; Mundstock, A.; Steinbach, F.; Caro, J. Hierarchical nanostructures of metal-organic frameworks applied in gas separating ZIF-8-on-ZIF-67 membranes. Chem. A Eur. J. 2018, 24, 5728–5733. [Google Scholar] [CrossRef]
- Han, Y.; Yang, C.; Zhou, Y.; Han, D.; Yan, H. Ionic liquid-hybrid molecularly imprinted material-filter solid-phase extraction coupled with HPLC for determination of 6-benzyladenine and 4-chlorophenoxyacetic acid in bean sprouts. J. Agric. Food Chem. 2017, 65, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Feng, P. Densities and viscosities of ionic liquid with organic solvents. Appl. Sci. 2020, 10, 8342. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Filipowiak, K.; Buchwald, T.; Nowicki, M. Microcapsules containing task-specific ionic liquids for Zn(II) and Cu(II) recovery from dilute aqueous solutions. Sep. Purif. Technol. 2020, 250, 1–18. [Google Scholar] [CrossRef]
- Tian, M.; Fang, L.; Yan, X.; Xiao, W.; Row, K.H. Determination of heavy metal ions and organic pollutants in water samples using ionic liquids and ionic liquid-modified sorbents. J. Anal. Methods Chem. 2019, 2019, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Tian, M.; Yan, X.; Xiao, W.; Row, K.H. Dual ionic liquid-immobilized silicas for multi-phase extraction of aristolochic acid from plants and herbal medicines. J. Chromatogr. A 2019, 1592, 31–37. [Google Scholar] [CrossRef]
- Fang, L.; Tian, M.; Yan, X.; Xiao, W. Isolation of aflatoxin B1 from moldy foods by solid-phase extraction combined with bifunctional ionic liquid-based silicas. J. Anal. Methods Chem. 2018, 2018, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.A.; Hasan, Z.; Jhung, S.H. Ionic liquids supported on metal-organic frameworks: Remarkable adsorbents for adsorptive desulfurization. Chem. A Eur. J. 2014, 20, 376–380. [Google Scholar] [CrossRef]
- Sarker, M.; Ahmed, I.; Jhung, S.H. Adsorptive removal of herbicides from water over nitrogen-doped carbon obtained from ionic liquid@ZIF-8. Chem. A Eur. J. 2017, 323, 203–211. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Hou, X.; Xu, T.; Tong, J.; Zhang, J.; Ye, B.; Liu, B. Porous liquid: A stable ZIF-8 colloid in ionic liquid with permanent porosity. Langmuir 2018, 34, 3654–3660. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Serpa, A.; Napolitano-Tabares, P.I.; Šulc, J.; Pacheco-Fernández, I.; Pino, V. Role of ionic liquids in composites in analytical sample preparation. Separations 2020, 7, 37. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Lee, W.-D. Self-assembled magnetic graphene supported ZIF-67 as a recoverable and efficient adsorbent for benzotriazole. Chem. Eng. J. 2016, 284, 1017–1027. [Google Scholar]
- Chan, C.; Liu, Y.; Pavlović, N.M.; Chan, W. Aristolochic acids: Newly identified exposure pathways of this class of environmental and food-borne contaminants and its potential link to chronic kidney diseases. Toxics 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Saffarionpour, S.; Tam, S.S.; Wielen, L.A.M.; Brouwer, E.; Ottens, M. Influence of ethanol and temperature on adsorption of flavor-active esters on hydrophobic resins. Sep. Purif. Technol. 2019, 210, 219–230. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, H.; Han, Y.; Bai, L.; Yan, H. On-line enrichment and determination of aristolochic acid in medicinal plants using a MOF-based composite monolith as adsorbent. J. Chromatogr. B 2020, 1159, 122343. [Google Scholar] [CrossRef]
- Du, X.; Wang, C.; Liu, J.; Zhao, X.; Zhong, J.; Li, Y.; Li, J.; Wang, P. Extensive and selective adsorption of ZIF-67 towards organic dyes: Performance and mechanism. J. Colloid Interface Sci. 2017, 506, 437–441. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, D.; Yang, W.; An, X.; Sun, A.; Fan, X.; Pan, Q. In situ modification of ZIF-67 with multi-sulfonated dyes for great enhanced methylene blue adsorption via synergistic effect. Microporous Mesoporous Mater. 2020, 303, 1–9. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Y.; Chen, J.; Xu, P.; Zhou, Y. Preparation of ionic liquid modified magnetic metal-organic frameworks composites for the solid-phase extraction of α–chymotrypsin. Talanta 2018, 182, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xiao, R.; Tang, J.; Zhu, Q.; Li, X.; Xiong, Y.; Wu, X. Preparation and adsorption properties of molecularly imprinted polymer via RAFT precipitation polymerization for selective removal of aristolochic acid I. Talanta 2017, 162, 415–422. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; Chen, Y.; Deng, Q.; Wang, S. Dummy molecularly imprinted silica materials for effective removal of aristolochic acid I from kaempfer dutchmanspipe root extract. Microchem. J. 2020, 152, 1–7. [Google Scholar] [CrossRef]
- Shu, H.; Chen, G.; Wang, L.; Cui, X.; Wang, Q.; Li, W.; Chang, C.; Guo, Q.; Luo, Z.; Fu, Q. Adenine-coated magnetic multiwalled carbon nanotubes for the selective extraction of aristolochic acids based on multiple interactions. J. Chromatogr. A 2020, 1627, 1–9. [Google Scholar] [CrossRef]
- Shu, H.; Ge, Y.; Xu, X.; Guo, P.; Luo, Z.; Du, W.; Chang, C.; Liu, R.; Fu, Q. Hybrid-type carbon microcoil-chitosan composite for selective extraction of aristolochic acid I from Aristolochiaceae medicinal plants. J. Chromatogr. A 2018, 1561, 13–19. [Google Scholar] [CrossRef]






| AAI in Plant (μg/g) | Spiked (μg/g) | Found (μg/g) | Relative Recovery (%) | RSD (%) | |
|---|---|---|---|---|---|
| Intra-Day | Inter-Day | ||||
| 20.0 | 2.0 20.0 200.0 | 21.2 ± 0.04 40.0 ± 0.03 219.1 ± 0.02 | 96.2 100.0 99.6 | 3.5 3.8 4.0 | 3.9 3.9 4.0 |
| Sorbent | Targets | Capacity (mg/g) | Recycles | LOD (μg/mL) | Recovery (%) | RSD (%) | Ref. |
|---|---|---|---|---|---|---|---|
| ZIF-67 | Malachite green | 240.0 | 4 | - | 95.0 | - | [11] |
| ZIF-67 | Organic dyes | 4.3–800.0 | - | - | 62.4–86.8 | - | [34] |
| Sulfonated ZIF-67 | Organic dyes | 94.3–1250.0 | 5 | - | 2.9–92.9 | - | [35] |
| ZIF-67@MWCNT–OH@IL-Fe3O4 | Chymotrypsin | 637.7 | 5 | - | ~86 | 0.1–1.2 | [36] |
| MIP | AAI | 1.3 | 6 | 0.06 | 91.5 | 4.2 | [37] |
| MIS | AAI | 8.1 | - | 0.04 | - | - | [38] |
| Sil@IM-BIM | AAI | 16.7 | - | - | 70.0–110.6 | 3.5–9.1 | [24] |
| SiO2@CNT/Fe3O4 | AAI | 24.5 | 5 | 0.05 | 92.7–98.4 | 1.7–3.9 | [39] |
| UIO-66-NH2@NMA | AAI | 35.4 | - | 0.1 | 91.1–106.5 | 0.9–6.1 | [33] |
| CMCs@CS | AAI | 77.72 | - | 0.1 | 73.6–77.7 | 0.8–4.5 | [40] |
| ZIF-67@EIM-MIM | AAI | 50.9 | 7 | 0.02 | 82.9–90.1 | 3.5–4.0 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Li, X.; Yan, X.; Tian, M. Solid-Phase Extraction of Aristolochic Acid I from Natural Plant Using Dual Ionic Liquid-Immobilized ZIF-67 as Sorbent. Separations 2021, 8, 22. https://0-doi-org.brum.beds.ac.uk/10.3390/separations8020022
Chen P, Li X, Yan X, Tian M. Solid-Phase Extraction of Aristolochic Acid I from Natural Plant Using Dual Ionic Liquid-Immobilized ZIF-67 as Sorbent. Separations. 2021; 8(2):22. https://0-doi-org.brum.beds.ac.uk/10.3390/separations8020022
Chicago/Turabian StyleChen, Pei, Xiaoman Li, Xuemin Yan, and Minglei Tian. 2021. "Solid-Phase Extraction of Aristolochic Acid I from Natural Plant Using Dual Ionic Liquid-Immobilized ZIF-67 as Sorbent" Separations 8, no. 2: 22. https://0-doi-org.brum.beds.ac.uk/10.3390/separations8020022