Silver(I) and Gold(I) Monothiocarbonate Complexes: Synthesis, Structure, Luminescence
Abstract
:1. Introduction
2. Results and Discussion
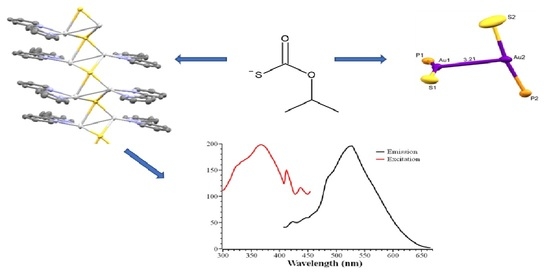
2.1. The Silver(I) Complex [{Ag4(SC(O)OiPr)2(2,2′-bpy)4}(PF6)2]n 1
2.2. The Gold(I) Complex [Au2{S(O)COiPr}2(dppe)]n 2
3. Materials and Methods
3.1. General
3.2. Synthesis of K[S(O)COiPr]
3.3. Synthesis of Complex [{Ag4(SC(O)OiPr)2(2,2′-bpy)4}(PF6)2]n 1
3.4. Synthesis of [Au2{S(O)COiPr}2(dppe)]n 2
3.5. Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Van Zyl, W.E. Dithiophosphonates and related P/S-type ligands of group 11 metals. Comments Inorg. Chem. 2010, 31, 13–45. [Google Scholar] [CrossRef]
- van Zyl, W.E.; Woollins, J.D. The coordination chemistry of dithiophosphonates: An emerging and versatile ligand class. Coord. Chem. Rev. 2013, 257, 718–731. [Google Scholar] [CrossRef]
- Mensforth, E.J.; Hill, M.R.; Batten, S.R. Coordination polymers of sulphur-donor ligands. Inorg. Chim. Acta 2013, 403, 9–24. [Google Scholar] [CrossRef]
- Belo, D.; Almeida, M. Transition metal complexes based on thiophene-dithiolene ligands. Coord. Chem. Rev. 2010, 254, 1479–1492. [Google Scholar] [CrossRef]
- Heard, P.J. Main Group Dithiocarbamate Complexes. In Progress in Inorganic Chemistry; Karlin, K.D., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; Chapter 1; Volume 53. [Google Scholar] [CrossRef]
- Hogarth, G. Transition Metal Dithiocarbamates: 1978–2003. In Progress in Inorganic Chemistry; Karlin, K.D., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; Chapter 2; Volume 53. [Google Scholar] [CrossRef]
- Oliveira, J.W.d.F.; Rocha, H.A.O.; de Medeiros, W.M.T.Q.; Silva, M.S. Application of Dithiocarbamates as Potential New Antitrypanosomatids-Drugs: Approach Chemistry, Functional and Biological. Molecules 2019, 24, 2806. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.S.; Yeo, C.I.; Tiekink, E.R.T.; Heard, P.J. Dithiocarbamate Complexes of Platinum Group Metals: Structural Aspects and Applications. Inorganics 2021, 9, 60. [Google Scholar] [CrossRef]
- Tercero, N.; Nagaraj, D.R.; Farinato, R. A Critical Overview of Dithiophosphinate and Dithiophosphate Interactions with Base Metal Sulfides and Precious Metals. Min. Metall. Explor. 2019, 36, 99–110. [Google Scholar] [CrossRef]
- Haiduc, I.; Sowerby, B.R.; Lu, S.F. Stereochemical aspects of phosphor-1,1-Dithiolato metal complexes (dithiophosphates, dithiophosphinates): Coordination patterns, molecular structures and supramolecular associations—I. Polvhedron 1995, 14, 3389–3472. [Google Scholar] [CrossRef]
- Sarker, J.C.; Hogarth, G. Dithiocarbamate Complexes as Single Source Precursors to Nanoscale Binary, Ternary and Quaternary Metal Sulfides. Chem. Rev. 2021, 121, 6057–6123. [Google Scholar] [CrossRef] [PubMed]
- Pillay, M.N.; van Zyl, W.E.; Liu, C.W. A construction guide for high-nuclearity (≥50 metal atoms) coinage metal clusters at the nanoscale: Bridging molecular precise constructs with the bulk material phase. Nanoscale 2020, 12, 24331–24348. [Google Scholar] [CrossRef]
- Liao, J.-H.; Chang, H.-W.; Li, Y.-J.; Fang, C.-S.; Sarkar, B.; van Zyl, W.E.; Liu, C.W. Anion templating from a silver(i) dithiophosphate 1D polymer forming discrete cationic and neutral octa- and decanuclear silver(i) clusters. Dalton Trans. 2014, 43, 12380–12389. [Google Scholar] [CrossRef]
- Dhayal, R.S.; Liao, J.-H.; Kahlal, S.; Wang, X.; Liu, Y.-C.; Chiang, M.-H.; Van Zyl, W.E.; Saillard, J.-Y.; Liu, C.W. [Cu32(H)20{S2P(OiPr)2}12]: The largest number of hydrides recorded in a molecular metal cluster by neutron diffraction. Chem.-Eur. J. 2015, 21, 8369–8374. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, T.J.; Ollengo, M.; Le Roux, L.; Pillay, M.N.; Staples, R.J.; Biros, S.M.; Wenderich, K.; Mei, B.; Van Zyl, W.E. Heterodimetallic Ferrocenyl Dithiophosphonate Complexes of Nickel(II), Zinc(II) and Cadmium(II) as Sensitizers for TiO2-Based Dye-Sensitized Solar Cells. ChemistrySelect 2019, 4, 7416–7424. [Google Scholar] [CrossRef]
- Mkumbuzi, E.; van Zyl, W.E. Synthesis and structures of zinc and cadmium bis(dithiophosphonate) complexes. J. Mol. Struct. 2021, 1226, 129338. [Google Scholar] [CrossRef]
- Yusuf, T.L.; Quadri, T.W.; Tolufashe, G.F.; Olasunkanmi, L.O.; Ebenso, E.E.; van Zyl, W.E. Synthesis and structures of divalent Co, Ni, Zn and Cd complexes of mixed dichalcogen and dipnictogen ligands with corrosion inhibition properties: Experimental and computational studies. RSC Adv. 2020, 10, 41967–41982. [Google Scholar] [CrossRef]
- Ayom, G.E.; Khan, M.D.; Ingsel, T.; Lin, W.; Gupta, R.K.; Zamisa, S.J.; Van Zyl, W.E.; Revaprasadu, N. Flexible Molecular Precursors for Selective Decomposition to Nickel Sulfide or Nickel Phosphide for Water Splitting and Supercapacitance. Chem.-Eur. J. 2020, 26, 2693–2704. [Google Scholar] [CrossRef]
- Pillay, M.N.; Liao, J.-H.; Liu, C.W.; Van Zyl, W.E. Aqueous Route to Stable Luminescent Tetranuclear Copper(I) Dithiophosphonate Clusters. Inorg. Chem. 2019, 58, 7099–7106. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Kishore, P.V.V.N.; Cyue, J.-Y.; Liao, J.-H.; Duminy, W.; van Zyl, W.E.; Liu, C.W. [Cu{SC(O)OiPr}]96: A Giant Self-Assembled Copper(I) Supramolecular Wheel Exhibiting Photoluminescence Tuning and Correlations with Dynamic Solvation and Solventless Synthesis. Inorg. Chem. 2021, 60, 8973–8983. [Google Scholar] [CrossRef]
- Cyue, J.-Y.; Kishore, P.V.V.N.; Liao, J.-H.; Lin, Y.-R.; Liu, C.W. Synthesis and characterization of CuI/AuI complexes derived from monothiocarbonate and tertiary phosphine ligands. Inorg. Chim. Acta 2017, 462, 97–105. [Google Scholar] [CrossRef]
- Murphy, C.N.; Winter, G. Monothiocarbonates and their oxidation to disulphides. Aust. J. Chem. 1973, 26, 755–760. [Google Scholar] [CrossRef]
- Ciborska, A.; Hnatejko, Z.; Kazimierczuk, K.; Mielcarek, A.; Wiśniewska, A.; Dołęga, A. Silver complexes stabilized by largesilanethiolate ligands—Crystal structures and luminescence properties. Dalton Trans. 2017, 46, 11097–11107. [Google Scholar] [CrossRef] [PubMed]
- Rogovoy, M.I.; Berezin, A.S.; Kozlova, Y.N.; Samsonenko, D.G.; Artem’Ev, A.V. A layered Ag(I)-based coordination polymer showing sky-blue luminescence and antibacterial activity. Inorg. Chem. Commun. 2019, 108, 107513. [Google Scholar] [CrossRef]
- Luo, S.-Q.; Wang, Q.; Quan, J.; Yang, M.; Wang, Y.; Zhang, X.; Chen, Z.-N. A sky-blue luminescent silver(I) complex with a one-dimensional zipper-like structure constructed with 2-diphenylphosphinopyridine and thiocyanate. Transit. Met. Chem. 2021, 46, 415–421. [Google Scholar] [CrossRef]
- Li, B.; Huang, R.-W.; Qin, J.-H.; Zang, S.-Q.; Gao, G.-G.; Hou, H.-W.; Mak, T.C.W. Thermochromic Luminescent Nest-Like Silver Thiolate Cluster. Chem.-Eur. J. 2014, 20, 12416–12420. [Google Scholar] [CrossRef]
- Rogovoy, M.I.; Samsonenko, D.G.; Rakhmanova, M.I.; Artem’Ev, A.V. Self-assembly of Ag(I)-based complexes and layered coordination polymers bridged by (2-thiazolyl)sulfides. Inorg. Chim. Acta 2019, 489, 19–26. [Google Scholar] [CrossRef]
- Chiu, T.-H.; Liao, J.-H.; Gam, F.; Chantrenne, I.; Kahlal, S.; Saillard, J.-Y.; Liu, C.W. All-selenolate-protected eight-electron platinum/silver nanoclusters. Nanoscale 2021, 13, 12143–12148. [Google Scholar] [CrossRef]
- Zhong, Y.J.; Liao, J.H.; Chiu, T.H.; Kahlal, S.; Lin, C.J.; Saillard, J.Y.; Liu, C.W. A Two-Electron Silver Superatom Isolated from Thermally Induced Internal Redox Reaction of A Silver(I) Hydride. Angew. Chem. Int. Ed. 2021, 60, 12712–12716. [Google Scholar] [CrossRef] [PubMed]
- Artem’Ev, A.V.; Shafikov, M.Z.; Schinabeck, A.; Antonova, O.V.; Berezin, A.S.; Bagryanskaya, I.Y.; Plusnin, P.E.; Yersin, H. Sky-blue thermally activated delayed fluorescence (TADF) based on Ag(i) complexes: Strong solvation-induced emission enhancement. Inorg. Chem. Front. 2019, 6, 3168–3176. [Google Scholar] [CrossRef]
- Potwana, F.S.W.; Pillay, M.N.; Staples, R.J.; Adeniyi, A.A.; Singh, P.; van Zyl, W.E. Silver(I) bis(phosphanylamino)naphthalene complexes: Synthesis, structures and density functional theory (DFT) calculations. Inorg. Chim. Acta 2021, 515, 120041. [Google Scholar] [CrossRef]
- Zhang, M.; Tan, Y.-L.; Chen, X.-R.; Yu, H.; Zhang, W.-H.; Lang, J.-P. A cationic [Ag12S12] cluster-based 2D coordination polymer and its dye composite with enhanced photocurrent and dielectric responses. Dalton Trans. 2019, 48, 8546–8550. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Schier, A. Argentophilic Interactions. Angew. Chem. Int. Ed. 2015, 54, 746–784. [Google Scholar] [CrossRef]
- Caballero-Muñoz, A.; Guevara-Vela, J.M.; Fernández-Alarcón, A.; Valentín-Rodríguez, M.A.; Flores-Álamo, M.; Rocha-Rinza, T.; Torrens, H.; Moreno-Alcántar, G. Structural Diversity and Argentophilic Interactions in Small Phosphine Silver(I) Thiolate Clusters. Eur. J. Inorg. Chem. 2021, 2021, 2702–2711. [Google Scholar] [CrossRef]
- Su, W.; Hong, M.; Weng, J.; Liang, Y.; Zhao, Y.; Cao, R.; Zhou, Z.; Chan, A.S.C. Tunable polymerization of silver complexes with organosulfur ligand: Counterions effect, solvent- and temperature-dependence in the formation of silver(I)-thiolate(and/or thione) complexes. Inorg. Chim. Acta 2002, 331, 8–15. [Google Scholar] [CrossRef]
- Li, M.-Q.; Zhao, M.; Bi, L.-Y.; Hu, Y.-Q.; Gou, G.; Li, J.; Zheng, Y.-Z. Two-Dimensional Silver(I)-Dithiocarboxylate Coordination Polymer Exhibiting Strong Near-Infrared Photothermal Effect. Inorg. Chem. 2019, 58, 6601–6608. [Google Scholar] [CrossRef] [PubMed]
- Brunner, H.; Hollman, A.; Zabel, M. Monomeric, dimeric and polymeric [Cp2MoH2] complexes with Ag-S bonds. J. Organomet. Chem. 2001, 630, 169–176. [Google Scholar] [CrossRef]
- Tan, Y.J.; Tan, Y.S.; Yeo, C.I.; Chew, J.; Tiekink, E.R.T. In vitro anti-bacterial and time kill evaluation of binuclear tricyclohexylphosphanesilver(I) dithiocarbamates, {Cy3PAg(S2CNRR′)}2. J. Inorg. Biochem. 2019, 192, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-B.; Gao, Q.; Zhang, C.-Y.; Sun, J.-H. Two three-dimensional silver(I) coordination architectures with pyridine-3,5-dicarboxylate: Luminescence and structural dependence on preparing conditions. J. Solid State Chem. 2009, 182, 1761–1766. [Google Scholar] [CrossRef]
- Chen, S.-C.; Cai, X.-X.; Zhang, Z.-H.; Yan, Q.; He, M.-Y.; Tian, F.; Gao, J.; Chen, Q. A Luminescent Silver(I) Metal-Organic Framework with New (4,6)-Connected Topology Based on Mixed Tetrachloroterephthalate and 2,2′-Bipyridine Ligands. Z. Anorg. Allg. Chem. 2013, 639, 1726–1730. [Google Scholar] [CrossRef]
- Onaka, S.; Yaguchi, M.; Yamauchi, R.; Ozeki, T.; Ito, M.; Sunahara, T.; Sugiura, Y.; Shiotsuka, M.; Nunokawa, K.; Horibe, M.; et al. The effect of carbon chain length of the diphosphine ligand on the aurophilic interaction. Synthesis and X-ray structural study for a series of Au(I) compounds with Ph2P–R–PPh2 and S-(CH2)n-py ligands. J. Organomet. Chem. 2005, 690, 57–68. [Google Scholar] [CrossRef]
- Li, J.; Zhu, X.-F.; Zhang, L.-Y.; Chen, Z.-N. Structures and luminescence properties of diethyldithiocarbamate-bridged polynuclear gold(i) cluster complexes with diphosphine/triphosphine. RSC Adv. 2015, 5, 34992–34998. [Google Scholar] [CrossRef]
- Zheng, A.-X.; Ren, Z.-G.; Li, L.-L.; Shang, H.; Li, H.-X.; Lang, J.-P. Reactions of a gold(i) thiolate complex [Au(Tab)2]2(PF6)2(Tab = 4-(trimethylammonio)benzenethiolate) with diphosphine ligands. Dalton Trans. 2011, 40, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Alcántar, G.; Romo-Islas, G.; Flores-Álamo, M.; Torrens, H. Aurophilicity vs. thiophilicity: Directing the crystalline supramolecular arrangement in luminescent gold compounds. New J. Chem. 2018, 42, 7845–7852. [Google Scholar] [CrossRef]
- Henderson, W.; Nicholson, B.K.; Tiekink, E.R.T. Synthesis, characterisation, supramolecular aggregation and biological activity of phosphine gold(I) complexes with monoanionic thiourea ligands. Inorg. Chim. Acta 2006, 359, 204–214. [Google Scholar] [CrossRef]
- Lee, R.; Igashira-Kamiyama, A.; Okumura, M.; Konno, T. Extraordinary Aggregation of Inorganic Anions in Chiral Metallosupramolecular Ionic Crystals. Bull. Chem. Soc. Jpn. 2013, 86, 908–920. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Römbke, P.; Schier, A.; Schmidbaur, H. Gold(I) organosulfinate and organosulfonate complexes. J. Chem. Soc. Dalton Trans. 2001, 17, 2482–2486. [Google Scholar] [CrossRef]
- Siasios, G.; Tiekink, E.R.T. Crystal Structures of Triorganophosphinegold(I) O-Cyclohexyldithiocarbonates: R3PAu(S2CO-c-C6H11), R = Et, Ph and c-Hexyl. Z. Krist.-Cryst. Mater. 1993, 204, 95–105. [Google Scholar] [CrossRef]
- Ho, S.Y.; Cheng, E.C.-C.; Tiekink, E.R.T.; Yam, V.W.-W. Luminescent Phosphine Gold(I) Thiolates: Correlation between Crystal Structure and Photoluminescent Properties in [R3PAu{SC(OMe) = NC6H4NO2-4}] (R = Et, Cy, Ph) and [(Ph2P-R-PPh2){AuSC(OMe) = NC6H4NO2-4}2] (R = CH2, (CH2)2, (CH2)3, (CH2)4, Fc). Inorg. Chem. 2006, 45, 8165–8174. [Google Scholar] [CrossRef]
- Fackler, J.P., Jr.; Van Zyl, W.E.; Prihoda, B.A. Gold Chalcogen Chemistry. In Gold: Progress in Chemistry, Biochemistry and Technology, 1st ed.; Schmidbaur, H., Ed.; John Wiley & Sons: Chichester, UK, 1999; Chapter 20; pp. 795–839. ISBN 0-471-97369-6. [Google Scholar]
- Maspero, A.; Kani, I.; Mohamed, A.A.; Omary, M.A.; Staples, R.J.; Fackler, J.P. Syntheses and Structures of Dinuclear Gold(I) Dithiophosphonate Complexes and the Reaction of the Dithiophosphonate Complexes with Phosphines: Diverse Coordination Types. Inorg. Chem. 2003, 42, 5311–5319. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Schier, A. A briefing on aurophilicity. Chem. Soc. Rev. 2008, 37, 1931–1951. [Google Scholar] [CrossRef]
- Kubas, J. Tetrakis(Acetonitrile)Copper(1+) Hexafluorophosphate(1-). Inorg. Synth. 1990, 28, 68–70. [Google Scholar]
- Uson, R.; Laguna, A.; Laguna, M.; Briggs, D.A.; Murray, H.H.; Fackler, J.P. (Tetrahydrothiophene) gold (I) or gold (III) complexes. Inorg. Synth. 1986, 26, 85. [Google Scholar]
- Demselben. Ueber die Darstellung und Eigenschaften des Kohlenoxysulfids. J. Prakt. Chem. 1887, 36, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Bruker. SAINT V4.043: Software for the CCD Detector System; Bruker: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G.M. SADABS; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. Program for Crystal Structure Determination. SHELXS 86. 1986. Available online: https://www.scienceopen.com/document?vid=a0637c27-7b3c-4348-92d7-edf82ad5b76d (accessed on 3 January 2022).
- Sheldrick, G.M. SHELXL-2014/7: Program for the Solution of Crystal Structures; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [Green Version]








| Complex | 1 | 2 |
|---|---|---|
| CCDC number | 2,126,277 | 2,126,328 |
| Chemical formula | C48H46Ag4N8O4S2 2(F6P) | C34H38Au2O4P2S2 |
| Mr | 1584.47 | 1030.63 |
| Crystal system | Orthorhombic | Monoclinic |
| Space group | Pca21 | P21/n |
| Temperature (K) | 150 | 150 |
| a (Å) | 28.2245 (7) | 11.7462 (9), |
| b (Å) | 7.1755 (2) | 39.197 (3), |
| c (Å) | 27.6678 (7) | 15.6529 (15) |
| α (°) | 90 | 90 |
| β (°) | 90 | 107.882 (4) |
| γ (°) | 90 | 90 |
| V (Å3) | 5603.4 (3) | 6858.7 (10) |
| Z | 4 | 8 |
| Ρcalcd (g cm−1) | 1.878 | 1.996 |
| µ (mm−1) | 1.600 | 8.798 |
| Tmin, Tmax | 0.575, 0.746 | 0.385, 0.746 |
| Reflections collected | 73,431 | 56,474 |
| Independent reflections | 13,261 | 15,085 |
| Observed reflections [I > 2σ(I)] | 12,168 | 11,582 |
| Rint | 0.029 | 0.037 |
| R[F2 > 2σ(F2)] | 0.025 | 0.085 |
| wR(F2) | 0.052 | 0.215 |
| S | 1.052 | 1.14 |
| Δρmax, Δρmin (e Å−3) | 1.00, −0.54 | 5.08, −3.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duminy, W.; Pillay, M.N.; van Zyl, W.E. Silver(I) and Gold(I) Monothiocarbonate Complexes: Synthesis, Structure, Luminescence. Inorganics 2022, 10, 19. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics10020019
Duminy W, Pillay MN, van Zyl WE. Silver(I) and Gold(I) Monothiocarbonate Complexes: Synthesis, Structure, Luminescence. Inorganics. 2022; 10(2):19. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics10020019
Chicago/Turabian StyleDuminy, Welni, Michael N. Pillay, and Werner E. van Zyl. 2022. "Silver(I) and Gold(I) Monothiocarbonate Complexes: Synthesis, Structure, Luminescence" Inorganics 10, no. 2: 19. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics10020019