Coordination Nature of 4-Mercaptoaniline to Sn(II) Ion: Formation of a One Dimensional Coordination Polymer and Its Decomposition to a Mono Nuclear Sn(IV) Complex
Abstract
:1. Introduction
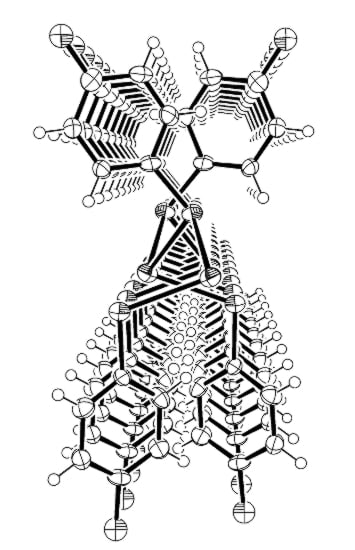
2. Results and Discussion
| Compound | 1 | 2 |
|---|---|---|
| Empirical formula | C12H15ClN2OS2Sn | C24H26Cl2N4S4Sn |
| Formula weight | 421.52 g mol−1 | 688.32 g mol−1 |
| Temperature | 273(2) K | 273(2) K |
| Wavelength | 0.71075 Å | 0.71075 Å |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P 2(1)/c | C 2/c |
| Unit cell dimensions | a = 17.328(14) Å; α = 90° | a = 31.03(2) Å; α = 90° |
| Volume | 1521(2) Å3 | 2871(4) Å3 |
| Z | 4 | 4 |
| Density (calculated) | 1.840 Mg/m3 | 1.593 Mg/m3 |
| Absorption coefficient | 2.122 mm−1 | 1.388 mm−1 |
| F(000) | 832 | 1384 |
| Crystal size | 0.29 × 0.16 × 0.03 mm3 | 0.23 × 0.15 × 0.14 mm3 |
| Theta range for data collection | 2.049 to 27.418° | 2.643 to 27.482° |
| Index ranges | −20 ≤ h ≤ 22, −15 ≤ k ≤ 15, −9 ≤ l ≤ 9 | −40 ≤ h ≤ 38, −10 ≤ k ≤ 10, −15 ≤ l ≤ 15 |
| Reflections collected | 8800 | 11610 |
| Independent reflections | 3443 [R(int) = 0.0726] | 3281 [R(int) = 0.0427] |
| Completeness to theta = 27.418° | 98.9% | 99.9% |
| Absorption correction | Semi-empirical from equivalents | Semi-empirical from equivalents |
| Max. and min. transmission | 1.00 and 0.870 | 1.000 and 0.829 |
| Refinement method | Full-matrix least-squares on F2 | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 3443/12/200 | 3281/0/211 |
| Goodness-of-fit on F2 | 1.064 | 1.074 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0536, wR2 = 0.1091 | R1 = 0.0321, wR2 = 0.0798 |
| R indices (all data) | R1 = 0.0974, wR2 = 0.1091 | R1 = 0.0373, wR2 = 0.0832 |
| Extinction coefficient | n/a | n/a |
| Largest diff. peak and hole | 0.806 and −1.030 e.Å−3 | 0.465 and −0.838 e.Å−3 |

| 1 | 2 | ||
|---|---|---|---|
| Bond lengths, Å | |||
| Sn(1)–S(1) | 2.563(3) | Sn(1)–S(1) | 2.399(1) |
| Sn(1)–S(2) | 2.702(2) | Sn(1)–S(2) | 2.395(1) |
| Bond angles, ° | |||
| S(1)–Sn(1)–S(2) | 92.28(6) | S(2)–Sn(1)–S(1) | 101.73(5) |

3. Experimental Section
3.1. General
3.2. Synthesis of [{Sn(µ-4-C6H4NH3S)(4-C6H4NH2S)}Cl∙H2O]n
3.3. Synthesis of [Sn(4-SC6H4NH2)2(4-SC6H4NH3)2Cl2]
3.4. X-ray Crystallography
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Osawa, M.; Matsuda, N.; Yoshii, K.; Uchida, I. Charge transfer resonance Raman process in surface-enhanced Raman scattering from p-aminothiophenol adsorbed on silver: Herzberg-Teller contribution. J. Phys. Chem. 1994, 98, 12702–12707. [Google Scholar] [CrossRef]
- Ho Choi, S.; Kim, B.; Frisbie, C.D. Electrical resistance of long conjugated molecular wires. Science 1994, 320, 1482–1486. [Google Scholar] [CrossRef]
- Johnson, S.R.; Evans, S.D.; Brydson, R. Influence of a terminal functionality on the physical properties of surfactant-stabilized gold nanoparticles. Langmuir 1998, 14, 6639–6647. [Google Scholar] [CrossRef]
- Zemlyanskii, N.N.; Borisova, I.V.; Kuznetsova, M.G.; Khrustalev, E.N.; Antipin, M.Y.; Ustynyuk, Y.A.; Lunin, E.E.; Eaborn, C.; Hill, M.S.; Smith, J.D. New Stable Germylenes, Stannylenes, and Related Compounds II. Bis(butylthio)tin(II) and ate-Complexes [(Me3Si)3CE(μ-SBu)2Li(THF)2] (E = Ge, Sn). Synthesis and Structure. Russ. J. Org. Chem. 2003, 39, 491–500. [Google Scholar] [CrossRef]
- Jetti, R.K.R.; Boese, R.; Thakur, T.S.; Vangala, V.R.; Desiraju, G.R. Proton transfer and N(+)–H···S(−) hydrogen bonds in the crystal structure of 4-aminothiophenol. Chem. Commun. 2004, 2526–2527. [Google Scholar] [CrossRef]
- Vidu, R.; Plapcianu, C.; Bartha, C. Multivalence Ce and Sn Oxide Doped Materials with Controlled Porosity for Renewable Energy Applications. Ind. Eng. Chem. Res. 2014, 53, 7829–7839. [Google Scholar] [CrossRef]
- Wang, H.; Rogach, A. Hierarchical SnO2 nanostructures: Recent advances in design, synthesis, and applications. Chem. Mater. 2013, 26, 123–133. [Google Scholar] [CrossRef]
- Tahar, R.; Ban, T. Tin doped indium oxide thin films: Electrical properties. J. Appl. Phys. 1998, 83, 2631–2645. [Google Scholar] [CrossRef]
- Barsan, N.; Schweizer-Berberich, M.; Gopel, W. Fundamental and practical aspects in the design of nanoscaled SnO2 gas sensors: A status report. Fresenius’ J. Anal. Chem. 1999, 365, 287–304. [Google Scholar] [CrossRef]
- Barth, S.; Hernandez-Ramirez, F.; Holmes, J.D.; Romano-Rodriguez, A. Synthesis and applications of one-dimensional semiconductors. Prog. Mater. Sci. 2010, 55, 563–627. [Google Scholar] [CrossRef]
- Hamberg, C. Evaporated tin-doped indium oxide films: Basic optical properties and applications to energy efficient windows. J. Appl. Phys. 1986, 60, 123–159. [Google Scholar] [CrossRef]
- Singh, O.M.; Devi, L.R. Stannous chloride as a versatile catalyst in organic synthesis. Mini. Rev. Org. Chem. 2013, 10, 84–96. [Google Scholar] [CrossRef]
- Thomas, J.M.; Johnson, B.F.G.; Raja, R.; Sankar, G.; Midgley, P.A. High-performance nanocatalysts for single-step hydrogenations. Acc. Chem. Res. 2003, 36, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Espinet, P.; Echavarren, A.M. The mechanisms of the Stille reaction. Angew. Chem. Int. Ed. Engl. 2004, 43, 4704–4734. [Google Scholar] [PubMed]
- Sun, Y.F.; Lei, F.C.; Gao, S.; Pan, B.; Zhou, J.F.; Xie, Y. Atomically thin tin dioxide sheets for efficient catalytic oxidation of carbon monoxide. Angew. Chem. Int. Ed. Engl. 2013, 52, 10569–10572. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Sun, G.; Xin, Q. Effect of alloying degree in PtSn catalyst on the catalytic behavior for ethanol electro-oxidation. Electrochim. Acta 2009, 54, 1511–1518. [Google Scholar] [CrossRef]
- Wu, J.; Risalvato, F.G.; Ma, S.; Zhou, X.-D. Electrochemical reduction of carbon dioxide III. The role of oxide layer thickness on the performance of Sn electrode in a full electrochemical cell. J. Mater. Chem. A 2014, 2, 1647. [Google Scholar] [CrossRef]
- Park, P.W.; Kung, H.H.; Kim, D.; Kung, M.C. Characterization of SnO2/Al2O3 Lean NOx Catalysts. J. Catal. 1999, 184, 440–454. [Google Scholar] [CrossRef]
- Batzill, M.; Diebold, U. The surface and materials science of tin oxide. Prog. Surf. Sci. 2005, 79, 47–154. [Google Scholar] [CrossRef]
- Ozin, G. New directions in tin sulfide materials chemistry. J. Mater. Chem. 1998, 8, 1099–1108. [Google Scholar] [CrossRef]
- Eichhöfer, A.; Jiang, J. 1-d-Tin(II) Phenylchalcogenolato Complexes ∞ 1[Sn(EPh)2] (E = S, Se, Te)—Synthesis, Structures, Quantum Chemical Studies and Thermal Behaviour. Eur. J. Inorg. Chem. 2010, 2010, 410–418. [Google Scholar] [CrossRef]
- Buttrus, N.H.; Suliman, M.M.; Al-Allaf, T.A.K. Synthesis of new Tin(IV) compounds of substituted diphenylsulfide derivatives and their complexes with some neutral ligands. Synth. React. Inorg. Met. Chem. 2001, 31, 837–848. [Google Scholar] [CrossRef]
- Wuyts, Y.; Vangindertaelen, A. The quadrivalence of tin and its mercaptides. Bull. Soc. Chim. Belg. 1921, 30, 323–328. [Google Scholar]
- Almagro, X.; Clegg, W. Schiff bases derived from mercury (II)-aminothiolate complexes as metalloligands for transition metals. J. Organomet. Chem. 2001, 623, 137–148. [Google Scholar] [CrossRef]
- Wang, L.S.; Zhang, J.J.; Du, W.X.; Hu, S.M.; Fu, R.-B.; Xia, S.-Q.; Wu, X.-T.; Xiang, S.-C.; Li, Y.-M.; Fu, Z.-Y. Syntheses and characterizations of (4-NH2C6H4S)4Sn and [(4-NH2C6H4S)2(4-NH3C6H4S)2Sn]Cl2. Chin. J. Struct. Chem. 2005, 24, 79–83. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burkett, E.S.; Siddiquee, T.A. Coordination Nature of 4-Mercaptoaniline to Sn(II) Ion: Formation of a One Dimensional Coordination Polymer and Its Decomposition to a Mono Nuclear Sn(IV) Complex. Inorganics 2014, 2, 652-659. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics2040652
Burkett ES, Siddiquee TA. Coordination Nature of 4-Mercaptoaniline to Sn(II) Ion: Formation of a One Dimensional Coordination Polymer and Its Decomposition to a Mono Nuclear Sn(IV) Complex. Inorganics. 2014; 2(4):652-659. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics2040652
Chicago/Turabian StyleBurkett, Eon S., and Tasneem A. Siddiquee. 2014. "Coordination Nature of 4-Mercaptoaniline to Sn(II) Ion: Formation of a One Dimensional Coordination Polymer and Its Decomposition to a Mono Nuclear Sn(IV) Complex" Inorganics 2, no. 4: 652-659. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics2040652