Tuning of Hula-Hoop Coordination Geometry in a Dy Dimer
Abstract
:1. Introduction

2. Results and Discussion
2.1. Synthesis of compounds 1 and 2
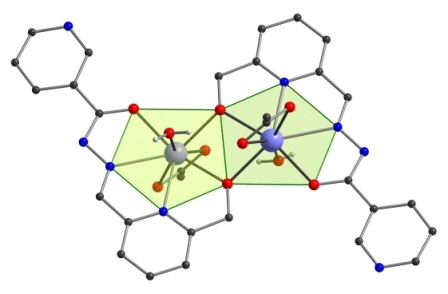
2.2. Molecular Structures of Compounds 1 and 2

2.3. Magnetic Properties of Compounds 1 and 2




2.4. Structure–Property Relationship
| Compounds | 1 | 2 | 3 * | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Bridging atoms | Alkoxide (O) | Alkoxide (O) | Hydrazone (O) | Hydrazone (O) | Hydrazone (O) | Phenoxide (O) |
| Geometry (Dy1) | hula hoop | hula hoop | pentagonal bipyramidal | hula hoop | hula hoop | hula hoop |
| Geometry (Dy1′/Dy2) | hula hoop | hula hoop | hula hoop | hula hoop | hula hoop | hula hoop |
| Donor atoms in cyclic ring | N2O3 | N2O3 | N2O3 | N2O3 | N2O3 | NO4 |
| coordination anions | Ac− | Ac− | Cl− or MeOH | NO3− | NO3− | NO3− |
| Coordination solvent | EtOH | H2O | Cl− or MeOH | H2O | MeOH | MeOH |
| Coupling | antiferro | antiferro | ferro | ferro | antiferro | ferro |
| daverage (Å) | 2.384 | 2.384 | 2.370 | 2.355 | 2.387 | |
| Dy–O–Dy (°) | 106.40° | 105.79° | 111.67° | 110.12° | 114.88° | 106.41° |
| Dy–Dy (Å) | 3.643 | 3.631 | 3.769 | 3.8258 | 3.9225 | |
| Field (Oe) | 2000 | 0 | 0 | 0 | 0 | 0 |
| Ueff (K) | 35.36 | 38.46 | 198, 150 | 69 | 41.29 | 56 |
| 1 | 2 | 3 (Dy1) | 3 (Dy2) | 4 | 5 |
|---|---|---|---|---|---|
| TDD-8 (D2d) | TDD-8 (D2d) | TDD-8 (D2d) | PBPY-7 (D5h) | TDD-8 (D2d) | BTPR-8 (C2v) |
| 2.45 | 2.75 | 2.22 | 1.17 | 2.47 | 3.80 |
3. Experimental Section
3.1. General Information
3.2. The Preparation of [Dy2(HL1)2(OAc)2(EtOH)2] (1) and [Dy2(L2)2(OAc)2(H2O)2]·2MeOH (2)
3.3. X-Ray Crystal Structures
3.4. Magnetic Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sessoli, R.; Powell, A.K. Strategies towards single molecule magnets based on lanthanide ions. Coord. Chem. Rev. 2009, 253, 2328–2341. [Google Scholar] [CrossRef]
- Osa, S.; Kido, T.; Matsumoto, N.; Re, N.; Pochaba, A.; Mrozinski, J. A Tetranuclear 3d-4f single molecule magnet: [CuIILTbIII(hfac)2]2. J. Am. Chem. Soc. 2003, 126, 420–421. [Google Scholar] [CrossRef] [PubMed]
- Sorace, L.; Benelli, C.; Gatteschi, D. Lanthanides in molecular magnetism: Old tools in a new field. Chem. Soc. Rev. 2011, 40, 3092–3104. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.D.; Long, J.R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2082. [Google Scholar] [CrossRef]
- Guo, Y.-N.; Xu, G.-F.; Guo, Y.; Tang, J. Relaxation dynamics of dysprosium(III) single molecule magnets. Dalton Trans. 2011, 40, 9953–9963. [Google Scholar] [CrossRef] [PubMed]
- Luzon, J.; Sessoli, R. Lanthanides in molecular magnetism: So fascinating, so challenging. Dalton Trans. 2012, 41, 13556–13567. [Google Scholar] [CrossRef] [PubMed]
- Palii, A.V.; Tsukerblat, B.; Klokishner, S.; Dunbar, K.R.; Clemente-Juan, J.M.; Coronado, E. Beyond the spin model: Exchange coupling in molecular magnets with unquenched orbital angular momenta. Chem. Soc. Rev. 2011, 40, 3130. [Google Scholar] [CrossRef] [PubMed]
- Palii, A.V.; Tsukerblat, B.S.; Clemente-Juan, J.M.; Coronado, E. Isotropic magnetic exchange between anisotropic Yb(III) ions. Study of Cs3Yb2Cl9 and Cs3Yb2Br9 crystals. Inorg. Chem. 2005, 44, 3984–3992. [Google Scholar] [CrossRef] [PubMed]
- Kostakis, G.E.; Hewitt, I.J.; Ako, A.M.; Mereacre, V.; Powell, A.K. Magnetic coordination clusters and networks: Synthesis and topological description. Philos. Trans. R. Soc. A 2010, 368, 1509–1536. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Habib, F.; Lin, P.-H.; Korobkov, I.; Enright, G.; Ungur, L.; Wernsdorfer, W.; Chibotaru, L.F.; Murugesu, M. Single-molecule magnet behavior for an antiferromagnetically superexchange-coupled dinuclear dysprosium(III) complex. J. Am. Chem. Soc. 2011, 133, 5319–5328. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-N.; Xu, G.-F.; Wernsdorfer, W.; Ungur, L.; Guo, Y.; Tang, J.K.; Zhang, H.-J.; Chibotaru, L.F.; Powell, A.K. Strong axiality and ising exchange interaction suppress zero-field tunneling of magnetization of an asymmetric Dy2 single-molecule magnet. J. Am. Chem. Soc. 2011, 133, 11948–11951. [Google Scholar]
- Lin, P.-H.; Sun, W.-B.; Yu, M.-F.; Li, G.-M.; Yan, P.-F.; Murugesu, M. An unsymmetrical coordination environment leading to two slow relaxation modes in a Dy2 single-molecule magnet. Chem. Commun. 2011, 47, 10993–10995. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-H.; Burchell, T.J.; Clérac, R.; Murugesu, M. Dinuclear dysprosium(III) single-molecule magnets with a large anisotropic barrier. Angew. Chem. Int. Ed. 2008, 4, 8848–8851. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Zhao, L.; Chen, P.; Guo, Y.-N.; Guo, Y.; Li, Y.-H.; Tang, J. Phenoxido and alkoxido-bridged dinuclear dysprosium complexes showing single-molecule magnet behaviour. Dalton Trans. 2012, 41, 2966–2971. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-N.; Chen, X.-H.; Xue, S.; Tang, J. Modulating magnetic dynamics of three Dy2 complexes through Keto-Enol tautomerism of the o-vanillin picolinoylhydrazone ligand. Inorg. Chem. 2011, 50, 9705–9713. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-N.; Chen, X.-H.; Xue, S.; Tang, J. Molecular assembly and magnetic dynamics of two novel Dy6 and Dy8 aggregates. Inorg. Chem. 2012, 51, 4035–4042. [Google Scholar]
- Runschke, C.; Meyer, G. [La2I2(OH)2(Dibenzo-18-Krone-6)2]I(I3), ein kationischer, dimerer in-cavity-Komplex mit Iodid und Triiodid als Anionen. Z. Anorg. Allg. Chem. 1997, 623, 1493–1495. [Google Scholar] [CrossRef]
- Ruiz-Martinez, A.; Casanova, D.; Alvarez, S. Polyhedral structures with an odd number of vertices: Nine-coordinate metal compounds. Chem. Eur. J. 2008, 14, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Kahn, M.L.; Sutter, J.-P.; Golhen, S.; Guionneau, P.; Ouahab, L.; Kahn, O.; Chasseau, D. Systematic investigations of the nature of the coupling between a Ln(III) ion (Ln = Ce(III) to Dy(III)) and its aminoxyl radical ligands. Structural and magnetic characteristics of a series of {Ln(organic radical)2} compounds and the related {Ln(nitrone)2} derivatives. J. Am. Chem. Soc. 2000, 122, 3413–3421. [Google Scholar]
- Kahn, M.L.; Ballou, R.; Porcher, P.; Kahn, O.; Sutter, J.-P. Analytical determination of the {Ln-aminoxyl radical} exchange interaction taking into account both the ligand-field effect and the spin-orbit coupling of the lanthanide ion (Ln = DyIII and HoIII). Chem. Eur. J. 2002, 8, 525–531. [Google Scholar] [CrossRef]
- Abbas, G.; Lan, Y.; Kostakis, G.E.; Wernsdorfer, W.; Anson, C.E.; Powell, A.K. Series of isostructural planar lanthanide complexes [LnIII4(μ3-OH)2(mdeaH)2(piv)8] with single molecule magnet behavior for the Dy4 analogue. Inorg. Chem. 2010, 49, 8067–8072. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.F.; Wang, X.T.; Liao, W.P.; Wang, X.W.; Deng, R.P.; Zhang, H.-J.; Gao, S. Thiacalix[4]arene-supported planar Ln4 (Ln = TbIII, DyIII) clusters: Toward luminescent and magnetic bifunctional materials. Inorg. Chem. 2009, 48, 11743–11747. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.-F.; Lin, P.-H.; Habib, F.; Aharen, T.; Murugesu, M.; Deng, Z.-P.; Li, G.-M.; Sun, W.B. Planar tetranuclear Dy(III) single-molecule magnet and its Sm(III), Gd(III), and Tb(III) analogues encapsulated by Salen-type and β-diketonate ligands. Inorg. Chem. 2011, 50, 7059–7065. [Google Scholar] [CrossRef] [PubMed]
- Tuna, F.; Smith, C.A.; Bodensteiner, M.; Ungur, L.; Chibotaru, L.F.; McInnes, E.J.L.; Winpenny, R.E.P.; Collison, D.; Layfield, R.A. A High anisotropy barrier in a sulfur-bridged organodysprosium single-molecule magnet. Angew. Chem. Int. Ed. 2012, 51, 6976–6980. [Google Scholar] [CrossRef] [PubMed]
- Habib, F.; Brunet, G.; Vieru, V.; Korobkov, I.; Chibotaru, L.F.; Murugesu, M. Significant enhancement of energy barriers in dinuclear dysprosium single-molecule magnets through electron-withdrawing effects. J. Am. Chem. Soc. 2013, 135, 13242–13245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Guo, Y.-N.; Tang, J. Recent advances in dysprosium-based single molecule magnets: Structural overview and synthetic strategies. Coord. Chem. Rev. 2013, 257, 1728–1763. [Google Scholar] [CrossRef]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide single-molecule magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- Habib, F.; Murugesu, M. Lessons learned from dinuclear lanthanide nano-magnets. Chem. Soc. Rev. 2013, 42, 3278–3288. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lillo, J.; Tomsa, A.R.; Li, Y.; Chamoreau, L.M.; Cremades, E.; Ruiz, E.; Barra, A.L.; Proust, A.; Verdaguer, M.; Gouzerh, P. Synthesis, crystal structure and magnetism of new salicylamidoxime-based hexanuclear manganese(III) single-molecule magnets. Dalton Trans. 2012, 41, 13668–13681. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, M.; Avnir, D. Continuous symmetry measures, V: The classical polyhedra. Inorg. Chem. 1998, 37, 5575–5582. [Google Scholar] [CrossRef] [PubMed]
- Casanova, D.; Cirera, J.; Llunell, M.; Alemany, P.; Avnir, D.; Alvarez, S. Minimal distortion paths in polyhedral rearrangements. J. Am. Chem. Soc. 2004, 126, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Cirera, J.; Ruiz, E.; Alvarez, S. Generalized interconversion coordinates. Chem. Eur. J. 2006, 12, 3162–3167. [Google Scholar] [CrossRef] [PubMed]
- Bagai, R.; Wernsdorfer, W.; Abboud, K.A.; Christou, G. Exchange-biased dimers of single-molecule magnets in OFF and ON states. J. Am. Chem. Soc. 2007, 129, 12918–12919. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Y.; Mereacre, V.; Anson, C.E.; Powell, A.K. Tuning of Hula-Hoop Coordination Geometry in a Dy Dimer. Inorganics 2016, 4, 2. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics4010002
Peng Y, Mereacre V, Anson CE, Powell AK. Tuning of Hula-Hoop Coordination Geometry in a Dy Dimer. Inorganics. 2016; 4(1):2. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics4010002
Chicago/Turabian StylePeng, Yan, Valeriu Mereacre, Christopher E. Anson, and Annie K. Powell. 2016. "Tuning of Hula-Hoop Coordination Geometry in a Dy Dimer" Inorganics 4, no. 1: 2. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics4010002