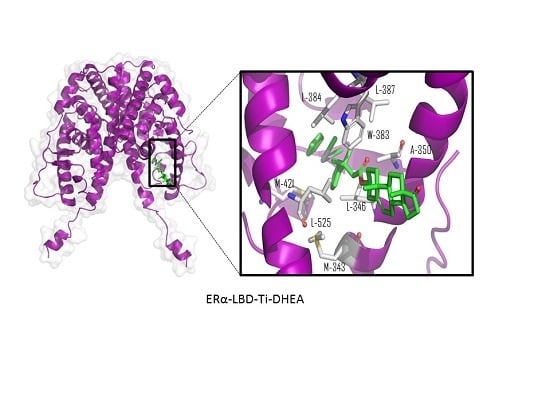
Steroid-Functionalized Titanocenes: Docking Studies with Estrogen Receptor Alpha
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Theoretical Calculations
3.2. Ligand Structure Optimization
3.3. Molecular Docking Protocols
3.3.1. Protein Preparation
3.3.2. ProtoMol Generation and Docking Calculations
3.4. Antiproliferative Studies
4. Concluding Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lorusso, D.; Petrelli, F.; Coinu, A.; Raspagliesi, F.; Barni, S. A systematic review comparing cisplatin and carboplatin plus paclitaxel-based chemotherapy for recurrent or metastatic cervical cancer. Gynecol. Oncol. 2014, 133, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Sandler, A.; Graham, C.; Baggstrom, M.; Herbst, R.; Zergebel, C.; Saito, K.; Jones, D. An open-label, multicenter, three-stage, phase II study of s-1 in combination with cisplatin as first-line therapy for patients with advanced non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef] [PubMed]
- Galanski, M.; Jakupec, M.A.; Keppler, B.K. Update of the preclinical situation of anticancer platinum complexes: Novel design strategies and innovative analytical approaches. Curr. Med. Chem. 2005, 12, 2075–2094. [Google Scholar] [CrossRef] [PubMed]
- Dempke, W.; Voigt, W.; Grothey, A.; Hill, B.T.; Schmoll, H.J. Cisplatin resistance and oncogenes—A review. Anticancer Drugs 2000, 11, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Köpf, H.; Köpf-Maier, P. Titanocene Dichloride—The First Metallocene with Cancerostatic Activity. Angew. Chem. Int. Ed. Engl. 1979, 18, 477–478. [Google Scholar] [CrossRef] [PubMed]
- Luemmen, G.; Sperling, H.; Luboldt, H.; Otto, T.; Ruebben, H. Phase II trial of titanocene dichloride in advanced renal-cell carcinoma. Cancer Chemother. Pharmacol. 1998, 42, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Kröger, N.; Kleeberg, U.R.; Mross, K.; Sass, G.; Hossfeld, D.K. Phase II clinical trial of titanocene dichloride in patients with metastatic breast cancer. Onkologie 2000, 23, 60–62. [Google Scholar] [CrossRef]
- Gao, L.M.; Vera, J.L.; Matta, J.; Meléndez, E. Synthesis and Cytotoxicity Studies of Steroid Functionalized Titanocenes as Potential Anticancer Drugs: Sex Steroids as Potential Vectors for Titanocenes. J. Biol. Inorg. Chem. 2010, 15, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Labrie, F. Dehydroepiandrosterone, androgens and the mammary gland. Gynecol. Endocrinol. 2006, 22, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Honda, A.; Matsuzak, Y.; Fukushima, S.; Tanaka, N.; Takagiwa, A.; Fujimoto, Y.; Miyazaki, H.; Salen, G. Anti-proliferative action of endogenous dehydroepiandrosterone metabolites on human cancer cell lines. Steroids 2003, 68, 73–83. [Google Scholar] [CrossRef]
- Hillard, E.A.; Vessières, A.; Jaouen, G. Ferrocene Functionalized Endrocrine Modulators as Anticancer Agents. Top. Organomet. Chem. 2010, 32, 81–117. [Google Scholar]
- Jordan, V.C. The role of tamoxifen in the treatment and prevention of breast cancer. Curr. Probl. Cancer 1992, 16, 129–176. [Google Scholar] [PubMed]
- Saturnino, C.; Sirignano, E.; Botta, A.; Sinicropi, M.S.; Caruso, A.; Pisano, A.; Lappano, R.; Maggiolini, M. Pasquale Longo, New titanocene derivatives with high antiproliferative activity against breast cancer cells. Bioorg. Med. Chem. Lett. 2014, 24, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Sirignano, E.; Saturnino, C.; Botta, A.; Sinicropi, M.S.; Caruso, A.; Pisano, A.; Lappano, R.; Maggiolini, M.; Longo, P. Synthesis, characterization and cytotoxic activity on breast cancer cells of new half-titanocene derivatives. Bioorg. Med. Chem. Lett. 2013, 23, 3458–3462. [Google Scholar] [CrossRef] [PubMed]
- Le Bideau, F.; Dagorne, S. Synthesis of Transition-Metal Steroid Derivatives. Chem. Rev. 2013, 113, 7793–7850. [Google Scholar] [CrossRef] [PubMed]
- Clearfield, A.; Warner, D.K.; Saltarriaga-Molina, C.H.; Ropal, R.; Bernal, I. Structural studies of complexes and their derivatives. The structure of bis(-cyclopentadienyl)titanium dichloride. Can. J. Chem. 1975, 53, 1622–1629. [Google Scholar] [CrossRef]
- Brzozowski, A.M.; Pike, A.C.; Dauter, Z.; Hubbard, R.E.; Bonn, T.; Engström, O.; Ohman, L.; Greene, G.L.; Gustafsson, J.A.; Carlquist, M. Molecular Basis of Agonism and Antagonism in the Oestrogen Receptor. Nature 1997, 389, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.N. Surflex: Fully automatic flexible molecular docking using a molecular similarity-based search engine. J. Med. Chem. 2003, 46, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Spyrakis, F.; Cozzini, P.; Bertoli, C.; Marabotti, A.; Kellogg, G.E.; Mozzarelli, A. Energetics of the protein-DNA-water interaction. BMC Struct. Biol. 2007, 7, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Vessieres, A.; Cabestaing, M.-A.P.C.; Claffey, J.; Dieckmann, S.; Hogan, M.; Müller-Bunz, H.; Strohfeldt, K.; Tacke, M. Proliferative and anti-proliferative effects of titanium- and iron-based metallocene anti-cancer drugs. J. Organomet. Chem. 2009, 694, 874–879. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.; Millam, J. GaussView, version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Sybyl-X Molecular Modeling Software Packages, version 2.0; TRIPOS Associates, Inc.: St. Louis, MO, USA, 2012.
- Mossman, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxycity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid Colorimetric Assay for Cell Growth and Survival. Modification to the Tetrazolium Dye Procedure Giving Improved Sensitivity and Reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Shankle, E.K.; Xu, W. Selectivity targeting estrogen receptors for cancer treatment. Adv. Drug Deliv. Rev. 2010, 62, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, C.; Cheng, G.J.; Zelada-Hedman, M.; Wärri, A.; Weihua, Z.; van Noorden, S.; Wahlstrom, T.; Coombes, R.C.; Warner, M.; Gustafsson, J. Estrogen receptor beta in breast cancer. End. Rel. Cancer 2002, 9, 1–13. [Google Scholar] [CrossRef]
- Helguero, L.A.; Faulds, M.H.; Gustafsson, J.Å.; Haldosén, L.-A. Estrogen receptors alfa (ERα) and beta (ERβ) differentially regulate proliferation and apoptosis of normal murine epithelial cell line HC11. Oncogene 2005, 24, 6605–6616. [Google Scholar] [CrossRef] [PubMed]
- Prossnitz, E.R.; Barton, M. Estrogen biology: New insights into GPER function and clinical opportunities. Mol. Cell. Endocrinol. 2014, 389, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Rosano, C.; Lappano, R.; Santolla, M.F.; Ponassi, M.; Donadini, A.; Maggiolini, M. Recent Advances in the Rationale Design of GPER Ligands. Curr. Med. Chem. 2012, 19, 6199–6206. [Google Scholar] [CrossRef] [PubMed]
- Arnatt, C.K.; Zhang, Y.G. G Protein-Coupled Estrogen Receptor (GPER) Agonist Dual Binding Mode Analyses Toward Understanding of its Activation Mechanism: A Comparative Modeling Approach. Mol. Inf. 2013, 32, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Barton, M. The membrane estrogen receptor GPER—Clues and questions. Steroids 2012, 77, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Albanito, L.; Madeo, A.; Lappano, R.; Vivacqua, A.; Rago, V.; Carpino, A.; Oprea, T.I.; Prossnitz, E.R.; Musti, A.M.; Ando, S.; et al. G Protein-Coupled Receptor 30 (GPR30) Mediates Gene Expression Changes and Growth Response to 17B-Estradiol and Selective GPR30 Ligand G-1 in Ovarian Cancer Cells. Cancer Res. 2007, 67, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.S.; Gunter, M.J.; Arend, R.C.; Li, M.; Arias-Pulido, H.; Prossnitz, E.R.; Goldberg, G.L.; Smith, H.O. Co-expression of GPR30 and ERbeta and their association with disease progression in uterine carcinosarcoma. Am. J. Obstet. Gynecol. 2010, 203, 242e1–242e5. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Ma, X.; Ostmann, A.B.; Das, S.K. GPER30 Activation Opposes Estrogen-Dependent Uterine Growth via Inhibition of Stromal ERK1/2 and Estrogen Receptor Alpha Phospholyration Signals. Endocrinology 2011, 152, 1434–1447. [Google Scholar] [CrossRef] [PubMed]






| Compound | Ti-Androsterone | Ti-Pregnenolone | Ti-Trans-Androsterone | Ti-DHEA |
|---|---|---|---|---|
| Distances (Å) | ||||
| Ti–C(Cp) ave. | 2.438 (22) | 2.441 (21) | 2.441 (21) | 2.439 (19) |
| Ti–C(Cp*) ave. | 2.46 (11) | 2.47 (10) | 2.47 (11) | 2.46 (11) |
| Ti–Cp centroid | 2.113 | 2.119 | 2.119 | 2.114 |
| Ti–Cp* centroid | 2.139 | 2.150 | 2.15 | 2.140 |
| Ti–Cl | 2.376, 2.380 | 2.378, 2.371 | 2.377, 2.371 | 2.379, 2.370 |
| Cp ave. | 1.431 (8) | 1.424 (8) | 1.424 (8) | 1.431 (8) |
| Cp* ave. | 1.433 (7) | 1.427 (7) | 1.427 (7) | 1.427 (10) |
| Angles (°) | ||||
| Bent angle | 132.76 | 131.92 | 131.84 | 132.72 |
| Cl–Ti–Cl | 97.40 | 97.58 | 97.62 | 97.62 |
| Ligand | Total Score (Surflex-Dock–log (Kd)) | Contact Residues/Type of Interaction | IC50 μM (Std) MCF-7 Cell Line |
|---|---|---|---|
| β-estradiol (Native ligand) | 7.4641 | Glu-353, Arg 394, His-524 (Hydrogen bonds); Leu-387, Met-388, Leu-391, Phe-404 (hydrophobic interactions) a | - |
| Ti-pregnenolone | 5.4054 | Met-388, Ile-424, Met-421, Leu-384, Leu-525, Ala-350, Trp-383, and Leu-354 (hydrophobic interactions) b | 20 (2) * |
| Ti-trans-androsterone | 8.1246 | Leu-354, Trp-383, Met-343, Thr-347, Ala-350, Leu-525, Leu-384, Met-421, Met-388, and Ile-424 (hydrophobic interactions) b | 40 (25) * |
| Ti-dehydroepiandrosterone | 9.5712 | Trp-383, Leu-525, Leu-384, Leu-387, Ala-350, Met-343, Met-421, and Leu-346 (hydrophobic interactions) b | 13 (2) * |
| Ti-androsterone | 5.7370 | Leu-428, Met-421,Ile-424, Leu-384, Met-388, Leu-346, Ala-350, Trp-383, and Thr-347 (hydrophobic interactions) b | 21 (5) * |
| Titanocene dichloride | 5.0383 | Leu-387, Leu-346, Leu-387, Leu-525,Phe-404, Met 421, and Met-388 (hydrophobic interactions) b | 570 (40) * |
| Pregnenolone | 5.3205 | Glu-353, Leu-387, Leu-525 (Hydrogen bonds); Leu-384, Ala-350, Leu-387, Leu-525, Leu-384, Met-421, Met-388 (hydrophobic interactions) b | 323 (29) * |
| Trans-androsterone | 8.0030 | His-524, Met-343, Glu-353, Leu-387 (Hydrogen bonds); Ala-350, Met-343, Leu-525, Leu-384, Leu-387, Met-388, Met-421 (hydrophobic interactions) b | 119 (9) |
| Dehydroepiandrosterone (DHEA) | 8.9881 | His-524, Met-343, Glu-353, Arg-394, Leu-387 (Hydrogen bonds); Leu-525, Ala-350, Leu-384, Leu-387, Met-388, Met-421 (hydrophobic interactions) b | 105 (5) |
| Androsterone | 7.2547 | His-524, Met-343 (Hydrogen bonds); Ala-350, Met-421, Leu-525, Leu-384, Met-343, Leu-387, Met-388 (hydrophobic interactions) b | 121 (10) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.M.; Maldonado, W.; Narváez-Pita, X.; Carmona-Negrón, J.A.; Olivero-Verbel, J.; Meléndez, E. Steroid-Functionalized Titanocenes: Docking Studies with Estrogen Receptor Alpha. Inorganics 2016, 4, 38. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics4040038
Gao LM, Maldonado W, Narváez-Pita X, Carmona-Negrón JA, Olivero-Verbel J, Meléndez E. Steroid-Functionalized Titanocenes: Docking Studies with Estrogen Receptor Alpha. Inorganics. 2016; 4(4):38. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics4040038
Chicago/Turabian StyleGao, Li Ming, Wilson Maldonado, Xiomara Narváez-Pita, José A. Carmona-Negrón, Jesus Olivero-Verbel, and Enrique Meléndez. 2016. "Steroid-Functionalized Titanocenes: Docking Studies with Estrogen Receptor Alpha" Inorganics 4, no. 4: 38. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics4040038