On Mineral Retrosynthesis of a Complex Biogenic Scaffold
Abstract
:1. Introduction
2. Results and Discussion
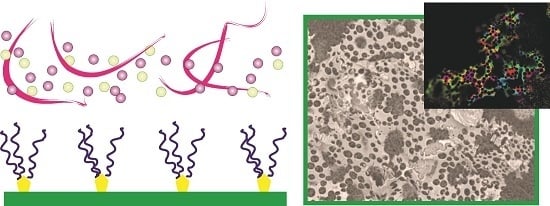
2.1. Architecture of the Organic–Inorganic Interface
2.2. Reconstructing the Mineral: Reference Experiments
2.3. Reconstructing the Mineral: Poly(acrylic acid)
2.4. Reconstructing the Mineral: Poly(aspartic acid)
2.5. Reconstructing the Mineral: Poly(4-styrenesulfonic acid-co-maleic acid)
2.6. Polymorph Selection during Scaffold-Assisted Mineralization
3. Materials and Methods
3.1. Materials
3.2. Staining and Swelling Studies
3.3. Gas Diffusion-Based Mineralization
4. Conclusions
- The formation of aragonite, which is typically induced by Mg2+ ions, is suppressed by the organic matrix of the egg shell. This hints that the biochemical environment presented by the egg membrane actively promotes calcite formation. Applying PAA 35000 during mineralization, the polymorph selectivity towards calcite is rendered ineffective possibly via charge screening and competitive ion-binding by additive molecules. Therefore, biomineral scaffolds and soluble additives operate synergistically during mineral polymorph selection.
- Mineralization additives with similar anionic groups can produce diverse mineral products in terms of shape, size, structure, as well as crystallographic orientation, due to the distinct molecular aspects of additives, such as conformation and chain-backbone chemistry. For instance, PASP leads to mammillae-associated nucleation of CaCO3 crystals. However, PAA 5000 leads to profuse surface mineralization, irrespective of mammillae distribution. Therefore, properties of additives, such as conformation and self-association, emerge as critical factors regulating mineralization.
- The molar mass of a mineralization additive profoundly affects mineral structure. For instance, unlike PAA 5000, PAA 35000 leads to microporous sheets formed via multiple nucleation events, in association with the egg membrane.
- Certain anionic additives lead to deformation and curvature of the egg organic matrix in the course of mineral growth. Phase transformation of attached mineral precursors induces surface-localized deformation of the membrane and subsequent membrane curvature. Similar mechanisms may operate during biogenic mineralization, determining the morphological aspects at larger length scales. To the best of our knowledge, this is the first report of mineralization-induced mechanical actuation.
- Primary and secondary nucleation are distinct due to the surface properties of bare and mineralized organic surfaces. In case of the collagen membrane, secondary wetting by liquid-like mineral precursors preferentially occurs on mineralized surfaces relative to the bare collagenous membrane. Underlying factors include the inhibitory activity of collagen towards mineral nucleation, distinct properties of organic and mineral surfaces and the induction of phase transformation by initially-formed crystalline particles. Therefore, in this context, the primary nucleation event is of utmost importance and lays the foundation of subsequent nucleation events encompassing mineral maturation.
- Properties of the mineral precursor phase, as well as subsequently-formed mineral products are determined by the nature of additive and co-additive species. For instance, on the inner surface of the egg membrane, in the presence of PASP, mineral particles with smooth surfaces suggestive of PILP droplets are formed. However, with the addition of Mg2+ ions, the particle surfaces are significantly coarse, suggesting a distinct mineralization process.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Weiner, S.; Addadi, L. Crystallization pathways in biomineralization. Annu. Rev. Mater. Res. 2011, 41, 21–40. [Google Scholar] [CrossRef]
- Lowenstam, H.A.; Weiner, S. On Biomineralization; Oxford University Press on Demand: Oxford, UK, 1989. [Google Scholar]
- Mann, S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry; Oxford University Press on Demand: Oxford, UK, 2001; Volume 5. [Google Scholar]
- Mao, L.-B.; Gao, H.-L.; Yao, H.-B.; Liu, L.; Cölfen, H.; Liu, G.; Chen, S.-M.; Li, S.-K.; Yan, Y.-X.; Liu, Y.-Y. Synthetic nacre by predesigned matrix-directed mineralization. Science 2016, 354, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Wagermaier, W.; Masic, A.; Kommareddy, K.P.; Bennet, M.; Manjubala, I.; Lee, S.-W.; Park, S.B.; Cölfen, H.; Fratzl, P. Self-assembly of amorphous calcium carbonate microlens arrays. Nat. Commun. 2012, 3, 725. [Google Scholar] [CrossRef] [PubMed]
- Heuer, A.; Fink, D.; Laraia, V.; Arias, J.; Calvert, P.; Kendall, K.; Messing, G.; Blackwell, J.; Rieke, P.; Thompson, D. Innovative materials processing strategies. Science 1992, 255, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Cölfen, H. Morphology control and molecular templates in biomineralization. In Biomineralization and Biomaterials: Fundamentals and Applications; Woodhead Publishing: Cambridge, UK, 2015; pp. 51–93. [Google Scholar]
- Hovden, R.; Wolf, S.E.; Holtz, M.E.; Marin, F.; Muller, D.A.; Estroff, L.A. Nanoscale assembly processes revealed in the nacroprismatic transition zone of pinna nobilis mollusc shells. Nat. Commun. 2015, 6, 10097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, A.; Cölfen, H. On the biophysical regulation of mineral growth: Standing out from the crowd. J. Struct. Biol. 2016, 196, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Cölfen, H. Mineralization and non-ideality: On nature’s foundry. Biophys. Rev. 2016, 8, 309–329. [Google Scholar] [CrossRef]
- Evans, J.S. “Liquid-like” biomineralization protein assemblies: A key to the regulation of non-classical nucleation. CrystEngComm 2013, 15, 8388–8394. [Google Scholar] [CrossRef]
- Rao, A.; Vásquez-Quitral, P.; Fernández, M.S.; Berg, J.K.; Sánchez, M.; Drechsler, M.; Neira-Carrillo, A.; Arias, J.L.; Gebauer, D.; Colfen, H. Ph-dependent schemes of calcium carbonate formation in the presence of alginates. Cryst. Growth Des. 2016, 16, 1349–1359. [Google Scholar] [CrossRef]
- Rao, A.; Seto, J.; Berg, J.K.; Kreft, S.G.; Scheffner, M.; Cölfen, H. Roles of larval sea urchin spicule SM50 domains in organic matrix self-assembly and calcium carbonate mineralization. J. Struct. Biol. 2013, 183, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Falini, G.; Fermani, S.; Abbott, C.; Moradian-Oldak, J. Supramolecular assembly of amelogenin nanospheres into birefringent microribbons. Science 2005, 307, 1450–1454. [Google Scholar] [CrossRef] [PubMed]
- Jain, G.; Pendola, M.; Rao, A.; Cölfen, H.; Evans, J.S. A model sea urchin spicule matrix protein self-associates to form mineral-modifying protein hydrogels. Biochemistry 2016, 55, 4410–4421. [Google Scholar] [CrossRef] [PubMed]
- Bera, T.; Ramachandrarao, P. A chicken’s egg as a reaction vessel to explore biomineralization. J. Bionic Eng. 2007, 4, 133–141. [Google Scholar] [CrossRef]
- Arias, J.L.; Arias, J.I.; Fernandez, M.S. Avian eggshell as a template for biomimetic synthesis of new materials. In Handbook of Biomineralization: Biomimetic and Bioinspired Chemistry; Wiley-VCH Verlag, GmBH & Co: Weinheim, Germany, 2007; Volume 2. [Google Scholar]
- Fernandez, M.S.; Araya, M.; Arias, J.L. Eggshells are shaped by a precise spatio-temporal arrangement of sequentially deposited macromolecules. Matrix Biol. 1997, 16, 13–20. [Google Scholar] [CrossRef]
- Carrino, D.A.; Dennis, J.E.; Wu, T.-M.; Arias, J.L.; Fernandez, M.S.; Rodriguez, J.P.; Fink, D.J.; Heuer, A.H.; Caplan, A.I. The avian eggshell extracellular matrix as a model for biomineralization. Connect. Tissue Res. 1996, 35, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Hincke, M.T.; Nys, Y.; Gautron, J.; Mann, K.; Rodriguez-Navarro, A.B.; McKee, M.D. The eggshell: Structure, composition and mineralization. Front. Biosci. 2012, 17, 1266–1280. [Google Scholar] [CrossRef]
- Demarchi, B.; Hall, S.; Roncal-Herrero, T.; Freeman, C.L.; Woolley, J.; Crisp, M.K.; Wilson, J.; Fotakis, A.; Fischer, R.; Kessler, B.M. Protein sequences bound to mineral surfaces persist into deep time. eLife 2016, 5, e17092. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.S.; Moya, A.; Lopez, L.; Arias, J.L. Secretion pattern, ultrastructural localization and function of extracellular matrix molecules involved in eggshell formation. Matrix Biol. 2001, 19, 793–803. [Google Scholar] [CrossRef]
- Nysl, Y.; Hincke, M.; Arias, J.; Garcia-Ruiz, J.; Solomon, S. Avian eggshell mineralization. Avian Poult. Biol. Rev. 1999, 10, 143–166. [Google Scholar]
- Hincke, M.T.; Nys, Y.; Gautron, J. The role of matrix proteins in eggshell formation. J. Poult. Sci. 2010, 47, 208–219. [Google Scholar] [CrossRef]
- Arias, J.L.; Fernandez, M.S.; Dennis, J.E.; Caplan, A.I. Collagens of the chicken eggshell membranes. Connect. Tissue Res. 1991, 26, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Panheleux, M.; Bain, M.; Fernandez, M.; Morales, I.; Gautron, J.; Arias, J.; Solomon, S.; Hincke, M.; Nys, Y. Organic matrix composition and ultrastructure of eggshell: A comparative study. Br. Poult. Sci. 1999, 40, 240–252. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.; Maček, B.; Olsen, J.V. Proteomic analysis of the acid-soluble organic matrix of the chicken calcified eggshell layer. Proteomics 2006, 6, 3801–3810. [Google Scholar] [CrossRef] [PubMed]
- Rose-Martel, M.; Du, J.; Hincke, M.T. Proteomic analysis provides new insight into the chicken eggshell cuticle. J. Proteom. 2012, 75, 2697–2706. [Google Scholar] [CrossRef]
- Arias, J.; Fernandez, M. Role of extracellular matrix molecules in shell formation and structure. World Poult. Sci. J. 2001, 57, 349–357. [Google Scholar] [CrossRef]
- Rao, A.; Fernández, M.S.; Cölfen, H.; Arias, J.L. Distinct effects of avian egg derived anionic proteoglycans on the early stages of calcium carbonate mineralization. Cryst. Growth Des. 2015, 15, 2052–2056. [Google Scholar] [CrossRef]
- Carrino, D.A.; Rodriguez, J.P.; Caplan, A.I. Dermatan sulfate proteoglycans from the mineralized matrix of the avian eggshell. Connect. Tissue Res. 1997, 36, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.; Passalacqua, K.; Arias, J.; Arias, J. Partial biomimetic reconstitution of avian eggshell formation. J. Struct. Biol. 2004, 148, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.; Siedler, F. The amino sequence of ovocleidin 17, a major protein of the avian eggshell calcified layer. IUBMB Life 1999, 47, 997–1007. [Google Scholar] [CrossRef]
- Chien, Y.-C.; Hincke, M.; Vali, H.; McKee, M. Ultrastructural matrix–mineral relationships in avian eggshell, and effects of osteopontin on calcite growth in vitro. J. Struct. Biol. 2008, 163, 84–99. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.S.; Escobar, C.; Lavelin, I.; Pines, M.; Arias, J.L. Localization of osteopontin in oviduct tissue and eggshell during different stages of the avian egg laying cycle. J. Struct. Biol. 2003, 143, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Maroudas, A.; Mizrahi, J.; Benaim, E.; Schneiderman, R.; Grushko, G. Swelling pressure of cartilage: Roles played by proteoglycans and collagen. In Mechanics of Swelling; Springer: Berlin, Germany, 1992; pp. 487–512. [Google Scholar]
- Olszta, M.; Douglas, E.; Gower, L. Scanning electron microscopic analysis of the mineralization of type i collagen via a polymer-induced liquid-precursor (PILP) process. Calcif. Tissue Int. 2003, 72, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Antebi, B.; Cheng, X.; Harris, J.N.; Gower, L.B.; Chen, X.-D.; Ling, J. Biomimetic collagen–hydroxyapatite composite fabricated via a novel perfusion-flow mineralization technique. Tissue Eng. Part C Methods 2013, 19, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, N.; Nassif, N.; Pinna, N.; Antonietti, M.; Gupta, H.S.; Cölfen, H. Retrosynthesis of nacre via amorphous precursor particles. Chem. Mater. 2005, 17, 6514–6516. [Google Scholar] [CrossRef]
- Hardikar, V.V.; Matijević, E. Influence of ionic and nonionic dextrans on the formation of calcium hydroxide and calcium carbonate particles. Colloids Surfaces A Physicochem. Eng. Asp. 2001, 186, 23–31. [Google Scholar] [CrossRef]
- Lakshminarayanan, R.; Loh, X.J.; Gayathri, S.; Sindhu, S.; Banerjee, Y.; Kini, R.M.; Valiyaveettil, S. Formation of transient amorphous calcium carbonate precursor in quail eggshell mineralization: An in vitro study. Biomacromolecules 2006, 7, 3202–3209. [Google Scholar] [CrossRef] [PubMed]
- Cusack, M.; Fraser, A.; Stachel, T. Magnesium and phosphorus distribution in the avian eggshell. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2003, 134, 63–69. [Google Scholar] [CrossRef]
- Wolf, S.L.; Jähme, K.; Gebauer, D. Synergy of Mg2+ and poly(aspartic acid) in additive-controlled calcium carbonate precipitation. CrystEngComm 2015, 17, 6857–6862. [Google Scholar] [CrossRef]
- Sancho-Tomás, M.; Fermani, S.; Reggi, M.; García-Ruiz, J.M.; Gómez-Morales, J.; Falini, G. Polypeptide effect on Mg2+ hydration inferred from CaCO3 formation: A biomineralization study by counter-diffusion. CrystEngComm 2016, 18, 3265–3272. [Google Scholar] [CrossRef]
- Berg, J.K.; Jordan, T.; Binder, Y.; Börner, H.G.; Gebauer, D. Mg2+ tunes the wettability of liquid precursors of CaCO3: Toward controlling mineralization sites in hybrid materials. J. Am. Chem. Soc. 2013, 135, 12512–12515. [Google Scholar] [CrossRef] [PubMed]
- Liao, B.; Qiao, H.; Zhao, X.; Bao, M.; Liu, L.; Zheng, C.; Li, C.; Ning, Z. Influence of eggshell ultrastructural organization on hatchability. Poult. Sci. 2013, 92, 2236–2239. [Google Scholar] [CrossRef] [PubMed]
- Parsons, A. Structure of the eggshell. Poult. Sci. 1982, 61, 2013–2021. [Google Scholar] [CrossRef]
- Arias, J.L.; Fernández, M.A.S. Polysaccharides and proteoglycans in calcium carbonate-based biomineralization. Chem. Rev. 2008, 108, 4475–4482. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, F.; Li, L.; Li, G.; He, W.; Linhardt, R.J. Compositional analysis and structural elucidation of glycosaminoglycans in chicken eggs. Glycoconj. J. 2014, 31, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Cha, W.I.; Hyon, S.H.; Ikada, Y. Transparent poly(vinyl alcohol) hydrogel with high water content and high strength. Die Makromol. Chem. 1992, 193, 1913–1925. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Mou, Y.; Tsai, T.W.-T.; Wu, Y.-J.; Lee, H.-K.; Huang, S.-J.; Chan, J.C. Calcium-43 NMR studies of polymorphic transition of calcite to aragonite. J. Phys. Chem. B 2012, 116, 14295–14301. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, D.; Cölfen, H.; Verch, A.; Antonietti, M. The multiple roles of additives in CaCO3 crystallization: A quantitative case study. Adv. Mater. 2009, 21, 435–439. [Google Scholar] [CrossRef]
- Huang, S.-C.; Naka, K.; Chujo, Y. A carbonate controlled-addition method for amorphous calcium carbonate spheres stabilized by poly(acrylic acid)s. Langmuir 2007, 23, 12086–12095. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Han, J.T.; Cho, K. Formation of amorphous calcium carbonate thin films and their role in biomineralization. Chem. Mater. 2004, 16, 1740–1746. [Google Scholar] [CrossRef]
- Sun, S.; Mao, L.B.; Lei, Z.; Yu, S.H.; Cölfen, H. Hydrogels from amorphous calcium carbonate and polyacrylic acid: Bio-inspired materials for “mineral plastics”. Angew. Chem. Int. Ed. 2016, 55, 11765–11769. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.L.P.; Caballero, L.; Melo, F.; Cölfen, H. Gel-like calcium carbonate precursors observed by in-situ AFM. Langmuir 2017, 33, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Oaki, Y.; Kajiyama, S.; Nishimura, T.; Imai, H.; Kato, T. Nanosegregated amorphous composites of calcium carbonate and an organic polymer. Adv. Mater. 2008, 20, 3633–3637. [Google Scholar] [CrossRef]
- Zimmerberg, J.; Kozlov, M.M. How proteins produce cellular membrane curvature. Nat. Rev. Mol. Cell Biol. 2006, 7, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S. Egg and Shell Quality; Wolfe Publishing Ltd.: London, UK, 1991. [Google Scholar]
- Bentov, S.; Weil, S.; Glazer, L.; Sagi, A.; Berman, A. Stabilization of amorphous calcium carbonate by phosphate rich organic matrix proteins and by single phosphoamino acids. J. Struct. Biol. 2010, 171, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Cölfen, H. Biomineralization: A crystal-clear view. Nat. Mater. 2010, 9, 960–961. [Google Scholar] [CrossRef] [PubMed]
- Nudelman, F.; Pieterse, K.; George, A.; Bomans, P.H.; Friedrich, H.; Brylka, L.J.; Hilbers, P.A.; de With, G.; Sommerdijk, N.A. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat. Mater. 2010, 9, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- Aizenberg, J.; Muller, D.A.; Grazul, J.L.; Hamann, D. Direct fabrication of large micropatterned single crystals. Science 2003, 299, 1205–1208. [Google Scholar] [CrossRef] [PubMed]
- Gower, L.B.; Odom, D.J. Deposition of calcium carbonate films by a polymer-induced liquid-precursor (PILP) process. J. Cryst. Growth 2000, 210, 719–734. [Google Scholar] [CrossRef]
- Murakami, D.; Jinnai, H.; Takahara, A. Wetting transition from the cassie–baxter state to the wenzel state on textured polymer surfaces. Langmuir 2014, 30, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Berg, J.K.; Kellermeier, M.; Gebauer, D. Sweet on biomineralization: Effects of carbohydrates on the early stages of calcium carbonate crystallization. Eur. J. Mineral. 2014, 26, 537–552. [Google Scholar] [CrossRef]
- Schenk, A.S.; Zope, H.; Kim, Y.-Y.; Kros, A.; Sommerdijk, N.A.; Meldrum, F.C. Polymer-induced liquid precursor (PILP) phases of calcium carbonate formed in the presence of synthetic acidic polypeptides—Relevance to biomineralization. Faraday Discuss. 2012, 159, 327–344. [Google Scholar] [CrossRef]
- Song, R.-Q.; Cölfen, H.; Xu, A.-W.; Hartmann, J.; Antonietti, M. Polyelectrolyte-directed nanoparticle aggregation: Systematic morphogenesis of calcium carbonate by nonclassical crystallization. Acs Nano 2009, 3, 1966–1978. [Google Scholar] [CrossRef] [PubMed]
- Song, R.Q.; Xu, A.W.; Antonietti, M.; Cölfen, H. Calcite crystals with platonic shapes and minimal surfaces. Angew. Chem. Int. Ed. 2009, 48, 395–399. [Google Scholar] [CrossRef] [PubMed]
- De Yoreo, J.J.; Gilbert, P.U.; Sommerdijk, N.A.; Penn, R.L.; Whitelam, S.; Joester, D.; Zhang, H.; Rimer, J.D.; Navrotsky, A.; Banfield, J.F. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 2015, 349. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.; Cölfen, H. Mineralization schemes in the living world: Mesocrystals. In New Perspectives on Mineral Nucleation and Growth; Springer: Berlin, Germany, 2017; pp. 155–183. [Google Scholar]
- Ehrlich, H.; Koutsoukos, P.G.; Demadis, K.D.; Pokrovsky, O.S. Principles of demineralization: Modern strategies for the isolation of organic frameworks: Part II. Decalcification. Micron 2009, 40, 169–193. [Google Scholar] [CrossRef] [PubMed]














© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, A.; Arias, J.L.; Cölfen, H. On Mineral Retrosynthesis of a Complex Biogenic Scaffold. Inorganics 2017, 5, 16. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics5010016
Rao A, Arias JL, Cölfen H. On Mineral Retrosynthesis of a Complex Biogenic Scaffold. Inorganics. 2017; 5(1):16. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics5010016
Chicago/Turabian StyleRao, Ashit, José L. Arias, and Helmut Cölfen. 2017. "On Mineral Retrosynthesis of a Complex Biogenic Scaffold" Inorganics 5, no. 1: 16. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics5010016