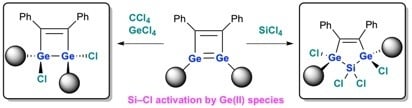
Synthesis of a Dichlorodigermasilane: Double Si–Cl Activation by a Ge=Ge Unit
Abstract
:1. Introduction
2. Results and Discussions
3. Materials and Methods
3.1. General Information
3.2. Experimental Details
3.2.1. Reaction of 1,2-Tbb2-1,2-Digermacyclobutadiene 1 with an Excess of SiCl4
3.2.2. Reaction of 1,2-Tbb2-1,2-Digermacyclobutadiene 1 with an Excess of GeCl4
3.2.3. Reaction of 1,2-Tbb2-1,2-Digermacyclobutadiene 1 with an Excess of CCl4
3.3. Computational Methods
3.4. X-ray Crystallographic Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Miller, R.D.; Michl, J. Polysilane High Polymers. Chem. Rev. 1989, 89, 1359–1410. [Google Scholar] [CrossRef]
- Amadoruge, M.L.; Weinert, C.S. Singly Bonded Catenated Germanes: Eighty Years of Progress. Chem. Rev. 2008, 108, 4253–4294. [Google Scholar] [CrossRef] [PubMed]
- Mochida, K.; Chiba, H. Synthesis, absorption characteristics and some reactions of polygermanes. J. Organomet. Chem. 1994, 473, 45–54. [Google Scholar] [CrossRef]
- Lickiss, P.D.; Smith, C.M. Silicon derivatives of the metals of groups 1 and 2. Coord. Chem. Rev. 1995, 145, 75–124. [Google Scholar] [CrossRef]
- Sekiguchi, A.; Lee, V.Y.; Nanjo, M. Lithiosilanes and their application to the synthesis of polysilane dendrimers. Coord. Chem. Rev. 2000, 210, 11–45. [Google Scholar] [CrossRef]
- Kyushin, S.; Matsumoto, H. Ladder Polysilanes. Adv. Organomet. Chem. 2003, 49, 133–166. [Google Scholar] [CrossRef]
- Weinert, C.S. Syntheses, structures and properties of linear and branched oligogermanes. Dalton Trans. 2009, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Drost, C.; Hitchcock, P.B.; Lappert, M.F. Unprecedented Oxidative Chlorosilylation Addition Reactions to a Diarylgermylene and -stannylene. Organometallics 2002, 21, 2095–2100. [Google Scholar] [CrossRef]
- Iwamoto, T.; Masuda, H.; Kabuto, C.; Kira, M. Trigermaallene and 1,3-Digermasilaallene. Organometallics 2005, 24, 197–199. [Google Scholar] [CrossRef]
- Kira, M.; Iwamoto, T.; Ishida, S.; Masuda, H.; Abe, T.; Kabuto, C. Unusual Bonding in Trisilaallene and Related Heavy Allenes. J. Am. Chem. Soc. 2009, 131, 17135–17144. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, T.; Sasamori, T.; Tokitoh, N. Highly Bent 1,3-Digerma-2-silaallene. Angew. Chem. Int. Ed. 2017, 56, 9920–9923. [Google Scholar] [CrossRef] [PubMed]
- Mallela, S.P.; Geanangel, R.A. New Cyclic and Acyclic Silicon–Germanium and Silicon–Germanium–Tin Derivatives. Inorg. Chem. 1994, 33, 1115–1120. [Google Scholar] [CrossRef]
- Mallela, S.P.; Geanangel, R.A. Preparation and structural characterization of new derivatives of digermane bearing tris(trimethylsilyl)silyl substituents. Inorg. Chem. 1991, 30, 1480–1482. [Google Scholar] [CrossRef]
- Ichinohe, M.; Sekiyama, H.; Fukaya, N.; Sekiguchi, A. On the Role of cis,trans-(t-Bu3SiGeCl)3 in the Reaction of GeCl2·Dioxane with Tri-tert-butylsilylsodium: Evidence for Existence of Digermanylsodium t-Bu3SiGe(Cl)2Ge(Cl)(Na)Sit-Bu3 and Digermene t-Bu3Si(Cl)Ge=Ge(Cl)Sit-Bu3. J. Am. Chem. Soc. 2000, 122, 6781–6782. [Google Scholar] [CrossRef]
- Fukaya, N.; Sekiyama, H.; Ichinohe, M.; Sekiguchi, A. Photochemical Generation of Chlorine-substituted Digermenes and Their Rearrangement to Germylgermylenes. Chem. Lett. 2002, 802–803. [Google Scholar] [CrossRef]
- Sekiguchi, A.; Ishida, Y.; Fukaya, N.; Ichinohe, M. The First Halogen-Substituted Cyclotrigermenes: A Unique Halogen Walk over the Three-Membered Ring Skeleton and Facial Stereoselectivity in the Diels-Alder Reaction. J. Am. Chem. Soc. 2002, 124, 1158–1159. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.Y.; Yasuda, H.; Ichinohe, M.; Sekiguchi, A. SiGe2 and Ge3: Cyclic Digermenes that Undergo Unexpected Ring-Expansion Reactions. Angew. Chem. Int. Ed. 2005, 44, 6378–6381. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.Y.; Yasuda, H.; Ichinohe, M.; Sekiguchi, A. Heavy cyclopropene analogues R4SiGe2 and R4Ge3 (R = SiMetBu2)—New members of the cyclic digermenes family. J. Organomet. Chem. 2007, 692, 10–19. [Google Scholar] [CrossRef]
- Wagler, J.; Brendler, E.; Langer, T.; Pöttgen, R.; Heine, T.; Zhechkov, L. Ylenes in the MII→SiIV (M=Si, Ge, Sn) Coordination Mode. Chem. Eur. J. 2010, 16, 13429–13434. [Google Scholar] [CrossRef] [PubMed]
- Al-Rafia, S.M.; Malcolm, A.C.; McDonald, R.; Ferguson, M.J.; Rivard, E. Trapping the Parent Inorganic Ethylenes H2SiGeH2 and H2SiSnH2 in the Form of Stable Adducts at Ambient Temperature. Angew. Chem. Int. Ed. 2011, 50, 8354–8357. [Google Scholar] [CrossRef] [PubMed]
- Katir, N.; Matioszek, D.; Ladeira, S.; Escudié, J.; Castel, A. Stable N-Heterocyclic Carbene Complexes of Hypermetallyl Germanium(II) and Tin(II) Compounds. Angew. Chem. Int. Ed. 2011, 50, 5352–5355. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.Y.; Ito, Y.; Yasuda, H.; Takanashi, K.; Sekiguchi, A. From Tetragermacyclobutene to Tetragermacyclobutadiene Dianion to Tetragermacyclobutadiene Transition Metal Complexes. J. Am. Chem. Soc. 2011, 133, 5103–5108. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Olmstead, M.M.; Power, P.P. Reactivity of Ar’GeGeAr’ (Ar’ = C6H3-2,6-Dipp2, Dipp = C6H3-2,6-iPr2) toward Alkynes: Isolation of a Stable Digermacyclobutadiene. J. Am. Chem. Soc. 2004, 126, 5062–5063. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, T.; Guo, J.-D.; Sasamori, T.; Karatsu, Y.; Furukawa, Y.; Ferao, A.E.; Nagase, S.; Tokitoh, N. Reaction of a Stable Digermyne with Acetylenes: Synthesis of a 1,2-Digermabenzene and a 1,4-Digermabarrelene. Bull. Chem. Soc. Jpn. 2016, 89, 1375–1384. [Google Scholar] [CrossRef]
- Tashkandi, N.Y.; Pavelka, L.C.; Caputo, C.A.; Boyle, P.D.; Power, P.P.; Baines, K.M. Addition of alkynes to digermynes: Experimental insight into the reaction pathway. Dalton Trans. 2016, 45, 7226–7230. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Jones, C.; Frenking, G. Reaction Mechanism of the Symmetry-Forbidden [2+2] Addition of Ethylene and Acetylene to Amido-Substituted Digermynes and Distannynes Ph2N-EE-NPh2, (E = Ge, Sn): A Theoretical Study. Chem. Eur. J. 2015, 21, 12405–12413. [Google Scholar] [CrossRef] [PubMed]
- Tillmann, J.; Meyer, L.; Schweizer, J.I.; Bolte, M.; Lerner, H.-W.; Wagner, M.; Holthausen, M.C. Chloride-Induced Aufbau of Perchlorinated Cyclohexasilanes from Si2Cl6: A Mechanistic Scenario. Chem. A-Eur. J. 2014, 20, 9234–9239. [Google Scholar] [CrossRef] [PubMed]
- Gaussian 09 Program; Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Ohtaki, T.; Ando, W. Dichlorodigermacyclobutanes and Digermabicyclo[2.2.0]hexanes from the Reactions of [Tris(trimethylsilyl)methyl]chlorogermylene with Olefins. Organometallics 1996, 15, 3103–3105. [Google Scholar] [CrossRef]
- Espenbetov, A.A.; Struchkov Yu, T.; Kolesnikov, S.P.; Nefedov, O.M. Crystal and Molecular Structure of Δ1,72,2,6,6,-Tetramethyl-4-thia-8,8,9,9-tetrachloro-8,9-digermabicyclo[5.2.0]nonene; The First Representative of 1,2-Digermacyclobutenes. J. Organomet. Chem. 1984, 275, 33–37. [Google Scholar] [CrossRef]
- Pangborn, A.B.; Giardello, M.A.; Grubbs, R.H.; Rosen, R.K.; Timmers, F.J. Safe and Convenient Procedure for Solvent Purification. Organometallics 2004, 15, 1518–1520. [Google Scholar] [CrossRef]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Spagna, R. SIR2004: An improved tool for crystal structure determination and refinement. J. Appl. Cryst. 2005, 38, 381–388. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugahara, T.; Tokitoh, N.; Sasamori, T. Synthesis of a Dichlorodigermasilane: Double Si–Cl Activation by a Ge=Ge Unit. Inorganics 2017, 5, 79. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics5040079
Sugahara T, Tokitoh N, Sasamori T. Synthesis of a Dichlorodigermasilane: Double Si–Cl Activation by a Ge=Ge Unit. Inorganics. 2017; 5(4):79. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics5040079
Chicago/Turabian StyleSugahara, Tomohiro, Norihiro Tokitoh, and Takahiro Sasamori. 2017. "Synthesis of a Dichlorodigermasilane: Double Si–Cl Activation by a Ge=Ge Unit" Inorganics 5, no. 4: 79. https://0-doi-org.brum.beds.ac.uk/10.3390/inorganics5040079